Research at Argonne helps Abbott Labs develop anti-HIV drug
Back in 1996, when the Advanced Photon Source at the U.S. Department of Energy's Argonne National Laboratory first turned on its brilliant beam of X-rays, scientists from around the world were excited by the possibilities. Now, 10 years later, one of those “possibilities” is saving thousands of lives.
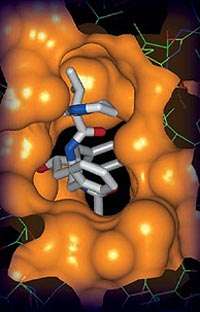
One of the early research projects undertaken at the Advanced Photon Source, which provides the Western Hemisphere's most brilliant X-ray beams, was an examination of the Acquired Immune Deficiency Syndrome (AIDS) Human Immunodeficiency Virus, or HIV.
Designing an effective anti-HIV drug requires very precise design: the drug must be highly target-specific so that it impacts only the point where the drug can be most effective. Also important are the lock-and-key requirements for drug designers. The design must fit the particular structure of the target molecule, while being flexible enough to accommodate changes in that structure.
Using X-ray crystallography, researchers found the points of attack of the HIV protease inhibitors – agents that block the breakdown of proteins. Protease inhibitors stop HIV from making new copies of itself by blocking the last step in the process, when the virus attempts to replicate.
Out of that discovery came the drug Kaletra®, now the most-prescribed drug in its class for AIDS therapy and a product of Abbott Laboratories, which was one of the earliest users of the Advanced Photon Source.
Abbott Labs is part of the Industrial Macromolecular Crystallography Association (IMCA), which operates one of the beamlines at the Advanced Photon Source through a contract with the Center for Advanced Radiation Sources at The University of Chicago. Researchers took a close-up view of the protein called the HIV protease, revealing the atomic details of how compounds interact with the protein.
“Kaletra is a clear example of the positive impact derived from research at our DOE-sponsored facility,” said Murray Gibson, Argonne associate laboratory director for scientific user facilities. “This premier national research facility provides the brightest X-ray beams in the Western Hemisphere to more than 5,000 scientists from around the United States and the world. These scientists come to the APS from universities, industry, medical schools, and other research institutions, bringing with them ideas for new discoveries in nearly every scientific discipline, from materials science to biology, chemistry, environmental and planetary science, and fundamental physics. They bring their ideas to the APS because our X-ray beams let them collect data in unprecedented detail and in amazingly short time frames. The knowledge they gain promises to have real and positive impact on our technologies, our health, our economy, and our fundamental understanding of the materials that make up our world.”
“The development of Kaletra is an excellent example of the critical path of drug discovery used by pharmaceutical companies,” said Jonathan Greer, vice president of research for Abbott Labs. “The first leg of the critical path is discovery, as we identify the target and generate leads toward appropriate drug properties; second is development, as the target is validated and the leads optimized, demonstrating the safety and efficacy of the drug candidate. The new technologies that help with this process, in addition to molecular crystallography and the X-ray techniques available at the APS, are high-throughput screening and chemistry, protein biochemistry, automation and the range of genomics and proteomics processes.”
One of the challenges facing structural biologists and indeed much of biology has been the slow pace, often measured in months or years, of determining the structures of biomolecules that are important for advances in energy, health and the environment. The use of the Department of Energy-funded user facility allows researchers to speed the process of structure determination, narrowing the gap between the current pace of discovery and the ability of scientists to have access to new information.
Because the research done at the Advanced Photon Source by Abbott Labs researchers was proprietary, the company reimbursed costs of using the facility for its research. In addition, as a part of the IMCA organization Abbott helped fund construction of the IMCA beamlines at the APS, which are openly available to non-IMCA researchers through the APS general user program. This collaboration between government and industry jointly advances scientific research.
Abbott researchers began clinical trials with Kaletra in the late 1990s and the longest clinical study of any HIV treatment – seven years – ended in late 2005 with data demonstrating that patients taking Kaletra in combination with other antiretroviral agents maintained an undetectable viral load (amount of virus in the blood) of less than 50 copies per milliliter, as measured by HIV RNA.
This finding, presented at the European AIDS Conference (EACS), demonstrated that most patients taking a Kaletra-based regimen as initial therapy for HIV infection showed sustained antiviral response. It is commonly remarked that Kaletra is a drug that helped turn a situation where patients were dying from AIDS to a situation where patients are living with AIDS.
"This impressive seven-year Kaletra data, which demonstrates sustainable treatment without resistance for most patients new to therapy, is a hallmark of the importance of research and development in the area of HIV," said Robert Murphy, M.D., practicing physician and professor, and director of clinical research in biodefense and infectious diseases, Division of Infectious Diseases, Northwestern University, Feinberg School of Medicine, Chicago.
Abbott has been a leader in HIV research since the early years of the epidemic. In 1985, the company developed the first licensed test to detect HIV antibodies in the blood and remains a leader in HIV diagnostics. Abbott retroviral and hepatitis tests are used to screen more than half of the world's donated blood supply.
Abbott is a global, broad-based health care company devoted to the discovery, development, manufacture and marketing of pharmaceuticals and medical products, including nutritionals, devices and diagnostics. The company employs 60,000 people and markets its products in more than 130 countries.
Source: by Catherine Foster, Argonne National Laboratory
















