FDA panel: Lower maximum daily dose of Tylenol
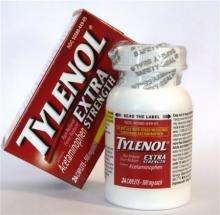
(AP) -- Government experts called for sweeping safety restrictions Tuesday on the most widely used painkiller, including reducing the maximum dose of Tylenol and eliminating prescription drugs such as Vicodin and Percocet.
The Food and Drug Administration assembled 37 experts to recommend ways to reduce deadly overdoses with acetaminophen, which is the leading cause of liver failure in the U.S. and sends 56,000 people to the emergency room annually. About 200 die each year.
"We're here because there are inadvertent overdoses with this drug that are fatal and this is the one opportunity we have to do something that will have a big impact," said Dr. Judith Kramer of Duke University Medical Center.
But over-the-counter cold medicines - such as Nyquil and Theraflu - that combine other drugs with acetaminophen can stay on the market, the panel said, rejecting a proposal to take them off store shelves.
The FDA is not required to follow the advice of its panels, though it usually does. The agency gave no indication when it would act on the recommendations.
In a series of votes Tuesday, the panel recommended 21-16 to lower the current maximum daily dose of over-the-counter acetaminophen from 4 grams, or eight pills of a medication such as Extra Strength Tylenol. They did not specify how much it should be lowered.
The panel also endorsed limiting the maximum single dose of the drug to 650 milligrams. That would be down from the 1,000-milligram dose, or two tablets of Extra Strength Tylenol.
A majority of panelists also said the 1,000-milligram dose should only be available by prescription.
The industry group that represents Johnson & Johnson, Wyeth and other companies defended the current dosing that appears on over-the-counter products.
"I think it's a very useful dose and one that is needed for treating chronic pain, such as people with chronic osteoarthritis," said Linda Suydam, president of the Consumer Healthcare Products Association.
The experts narrowly ruled that prescription drugs that combine acetaminophen with other painkilling ingredients should be eliminated. They cited FDA data indicating that 60 percent of acetaminophen-related deaths are related to prescription products.
But some on the panel opposed a sweeping withdraw of products that are widely used to control severe, chronic pain. Prescription acetaminophen combination drugs were prescribed 200 million times last year, according to the FDA.
"To make this shift without very clear understanding of the implications on the management of pain would be a huge mistake," said Dr. Robert Kerns of Yale University.
If the drugs stay on the market, they should carry a black box warning, the most serious safety label available, the panel decided.
"If we don't eliminate the combination products we should at least lower the levels of acetaminophen contained in those medicines," said Sandra Kewder, FDA's deputy director for new drugs, summarizing the panel's vote.
Percocet and similar treatments combine acetaminophen with more powerful pain relieving narcotics, such as oxycodone.
If the combination products are eliminated, the acetaminophen and the other ingredients could be prescribed separately. In effect, patients would take two pills instead of one, and be more aware of the acetaminophen they are consuming.
Vicodin is marketed by Abbott Laboratories, while Percocet is marketed by Endo Pharmaceuticals. Both painkillers also are available in cheaper generic versions.
"The panel recommending banning Vicodin and Percocet seems a little draconian," said Les Funtleyder, an analyst for Miller Tabak & Co.
Drug companies avoided the most damaging potential outcome with the defeat of proposal to pull NyQuil and other over-the-counter cold and cough medicines that combine acetaminophen with other drugs.
These drugs can be dangerous when taken with Tylenol or other drugs containing acetaminophen, according to the FDA, but cause only 10 percent of acetaminophen-related deaths.
"I don't think we should be advocating a solution to a problem that really is not there," said Dr. Osemwota Omoigui, of the Los Angeles pain clinic.
A recall of combination cold medicines would have cost manufacturers hundreds of millions of dollars in revenue. Total sales of all acetaminophen drugs reached $2.6 billion last year, with 80 percent of the market comprised of over-the-counter products, according to IMS Health, a health care analysis firm.
"The acetaminophen people dodged a bullet," said Erik Gordon, a University of Michigan business professor who studies the biomedical industry.
Even with the lower daily dosage recommendation, consumers will likely keep taking as many pills as they think they need to ease their pain, Gordon said.
Analyst Steve Brozak of WBB Securities said the panel votes were a "shot across the bow" of the pharmaceutical industry.
"This basically puts more government oversight into something that heretofore has been less than present," Brozak said.
---
AP Business writers Stephen Manning and Donna Borak contributed to this report.
©2009 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.