Inner workings of molecular thermostat point to pathways to fight diabetes, obesity
Best known as the oxygen-carrying component of hemoglobin, the protein that makes blood red, heme also plays a role in chemical detoxification and energy metabolism within the cell. Heme levels are tightly maintained, and with good reason: Too little heme prevents cell growth and division; excessive amounts of heme are toxic.
Researchers at the University of Pennsylvania School of Medicine have discovered a molecular circuit involving heme that helps maintain proper metabolism in the body, providing new insights into metabolic disorders such as obesity and diabetes.
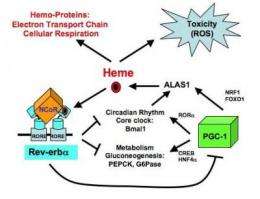
The work builds on 2007 findings from the same team, led by Mitchell Lazar, MD, PhD, Director of Penn's Institute for Diabetes, Obesity, and Metabolism, showing that a protein called Rev-erbα coordinates the daily cycles of heme. The new research, published online in Genes & Development, makes it clear that Rev-erbα, by controlling the production of heme, also plays a key role in maintaining the body's correct metabolism. This happens through a molecular pathway that allows the cell to monitor and adjust internal heme levels, creating more when heme levels fall, and slowing it down when levels rise.
The circuit is a negative feedback loop, with Rev-erbα as its central component, explains Lazar. "Rev-erbα is a thermostat for heme." When heme levels are high, Rev-erbα is activated, reducing heme, which leads the cell back towards a normal state. On the other hand, when heme levels are low, Rev-erbα activity is low, and this permits the cell to make more heme, again leading back toward a normal state. Maintaining this stasis allows energy metabolism to occur but avoids harm to the cell due to excessive levels of heme.
Understanding the control of heme levels is likely to be relevant to several diseases. For example, obesity is a condition where fat tissue builds up due to low energy expenditure relative to energy intake. Proteins such as Rev-erbα that help maintain a cell's proper metabolism and energy balance point to their role in such metabolic disorders as obesity and diabetes and suggest ways to intervene.
Rev-erbα is a transcription factor, a protein that binds to DNA in front of, or within, genes to alter their expression. Rev-erbα acts as repressor of gene expression, that is, gene expression goes down when it binds to DNA.
Lazar has been studying the protein for nearly 20 years, yet he never really knew how it worked. What he did know was that, as a member of a family of nuclear receptor proteins, Rev-erbα could bind DNA and likely had an intracellular binding partner.
Typical nuclear receptor proteins are like sensors, registering a specific molecular event and responding accordingly, generally by altering gene expression patterns. So, Lazar asked, "What is the purpose of having a system that responds to changes in cellular heme levels?" He hypothesized that the sensor could act to regulate heme itself.
Working with cultured human and mouse cells his team, led by first author, graduate student Nan Wu, monitored heme levels as Rev-erbα abundance changed. What they found confirmed the protein's role in heme regulation: when overexpressed, heme levels dropped; when suppressed, heme levels rose.
"That was consistent with the hypothesis," says Lazar. "The question was, how does heme do this?"
To figure that out, the team looked for Rev-erbα binding sites within the sequences of genes known to control heme biosynthesis and found one in PGC-1α, a transcription factor that stimulates the production of heme. Since Rev-erb activity is controlled by heme itself, the net effect is that, as heme levels rise, PGC-1α gets repressed, and heme synthesis drops off.
The team also demonstrated the physiological consequence of disrupting this pathway. "We reasoned, if heme levels get too low, cells won't like it," Lazar says, "and they don't: They stop growing, and they reduce their oxygen consumption in a manner consistent with the role of heme being used to make ATP," a form of cellular energy.
Lazar states that, "Up until now, no one knew there even was a mechanism for keeping heme levels in this narrow range. We've shown that it exists and have defined molecular players that make it work."
In so doing, he and his team have linked heme biosynthesis with both energy metabolism and the body's internal clock. Rev-erbα is a negative regulator of genes involved in energy metabolism. It also, along with PGC-1α and heme, rises and falls over a 24-hour period and even regulates some of the cogs within the clock itself.
Now the question is, can this pathway be exploited in the clinic. Lazar's team showed that downregulating heme stifled cell division and metabolism, while upregulating heme enhanced them. It therefore is possible, Lazar says, that by pharmacologically "tickling" Rev-erbα or its other cellular partners to believe the cell has more or less heme than it actually does, researchers may be able to either boost or suppress metabolism accordingly, opening the door to potential therapies for cancer and obesity.
Source: University of Pennsylvania School of Medicine (news : web)