Putting top brains to the test with Alzheimer's protein
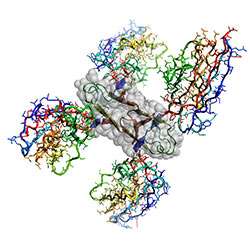
CSIRO scientists and collaborators have made major inroads in cracking a structure of the protein thought to cause Alzheimer’s disease.
Alzheimer’s disease is one of the fastest growing diseases in Australia and the most common form of dementia. The number of people with dementia is expected to rise from 300,000 to 1.13 million by 2050.
Alzheimer’s disease is associated with the development of a toxic protein in the brain known as amyloid beta. The amyloid beta protein rapidly self-assembles in the brain and builds up to form plaques which are a hallmark of the disease.
It is thought that the accumulation of small amyloid beta aggregates and plaques in the brain disrupt connections within the hippocampus (the area of the brain involved in memory), causing loss of neuron function and symptoms associated with Alzheimer’s disease such as memory loss.
There is no cure for Alzheimer’s disease, but determining the structure of amyloid beta protein is a vital step towards understanding why aggregates and plaques occur – knowledge that could result in the development of new treatments.
“Before we can understand the processes involved in the deterioration of the brain, we must determine the molecular shape of the damaging protein,” Director of CSIRO’s Preventative Health Flagship, Professor Richard Head said.
“Until now this has proved incredibly difficult because of the protein’s propensity to self assemble and clump together.”
Using a highly imaginative approach, CSIRO’s Dr. Jose Varghese and his team devised a way of capturing the protein in a crystal long enough to enable its analysis at the Australian Synchrotron through x-ray crystallography, solving one of a number of potential amyloid beta small aggregates that may play a role in the development of Alzheimer’s disease.
Dr. Varghese – who played a major role in the development of the current anti-influenza drugs – said the CSIRO team was the first to successfully crystallize the part of amyloid beta that forms plaque by fusing it to a shark antibody to prevent clumping.
“This enabled a structure to be resolved to atomic resolution thus providing an insight into the early molecular processes that occur in Alzheimer’s disease,” Dr. Varghese said.
“The discovery of the amyloid beta’s structure offers a molecular target for early detection of Alzheimer’s and may provide a vital step towards developing a drug to treat the disease.”
A co-author of the paper, Professor Colin Masters from the Mental Health Research Institute at the University of Melbourne, said the structure of the amyloid beta has been sought for the past 25 years.
“This discovery provides one of many possible structures, but it is a starting point for discovering drugs which might be used to interfere with the accumulation of the amyloid beta in Alzheimer’s disease,” Professor Masters said.
A description of the protein’s structure, and the novel approaches used by CSIRO scientists Dr. Victor Streltsov and Dr. Stewart Nuttall to reveal it, have been published in the latest edition of the Journal of Neurosciences.















