One for you, one for me: Researchers gain new insight into the chromosome separation process
Each time a cell divides -- and it takes millions of cell divisions to create a fully grown human body from a single fertilized cell -- its chromosomes have to be accurately divvied up between both daughter cells. Researchers at the Stowers Institute for Medical Research used, ironically enough, the single-celled organism Saccharomyces cerevisiae -- commonly known as baker's yeast -- to gain new insight into the process by which chromosomes are physically segregated during cell division.
In a study published in the Nov. 17, 2011 issue of PLoS Genetics, they demonstrate that a protein known as Mps3 not only ensures that cells have two functional spindle pole bodies, which generate the mitotic spindle apparatus that helps pull the chromosomes apart, but also that both spindle pole bodies are properly anchored in the nuclear membrane.
"When you enter mitosis, you need to have two spindle pole bodies on which you can pull the chromosomes. If you don't, the probability of errors in chromosome segregation increases exponentially," explains Jaspersen, adding that, "even small mistakes can lead to birth defects, genetic instability and cancer."
Normally, cells have only a single spindle pole body, but in preparation for cell division, the spindle pole body has to duplicate itself – just as the genome does. "We know a whole lot about how DNA copies itself, but we don't know much about how spindle pole bodies duplicate themselves," says Jaspersen.
Unlike DNA molecules, which serve as templates for the production of identical copies, the spindle pole body is a large protein structure composed of soluble proteins and so-called integral membrane proteins, which are anchored in the nuclear envelope. The duplication process of the lone spindle pole body begins when soluble proteins coalesce on the nuclear envelope followed by their insertion into the lipid bi-layer located next to the original spindle pole body. Insertion probably requires the integral membrane proteins of the spindle pole body and results in a second functional spindle pole body.
While many genes are known to be required for spindle pole body duplication, the best studied are perhaps the conserved family of SUN-domain proteins. The SUN-protein homolog in yeast is Mps3, an integral membrane component of the spindle pole body required for early steps in the duplication process.
"Cells with little or no functional Mps3 do not divide, and have only one spindle pole body and one half of the mitotic spindle," explains Jaspersen. "We were interested in how a spindle pole body gets inserted into the nuclear envelope, what modifications of this double lipid bi-layer membrane have to occur to facilitate insertion, and what is Mps3's role in all of this?"
To better understand the function of Mps3 in spindle pole body duplication, Jaspersen's team, led by co-first authors Jennifer Friederichs and Suman Ghosh, Ph.D., mutated specific regions of the Mps3 gene and then expressed the mutated genes in yeast. For most of the mutants, mitosis seemed normal. That wasn't the case, however, with one particular novel mutant, MPS3-G186K, which has a small, "point" mutation in the so-called P-loop region.
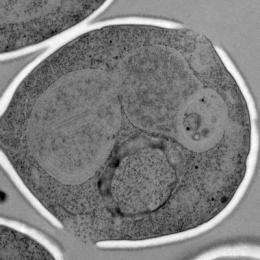
The researchers next used high-resolution electron microscopy and various markers, including ones that can distinguish uninserted and inserted spindle pole bodies. What they saw was that, although their DNA had been duplicated, cells expressing this particular Mps3 mutant had multiple duplication defects, including blocking insertion of the spindle pole body into the nuclear envelope.
What was most striking, however, was that nearly every cell examined had nuclear membranes that were, essentially, overgrown—with two to eight layers of nuclear envelope, and multiple lobes and extensions -- instead of a simple spherical structure. Importantly, the effect seemed specific in that the other membrane-based organelles appeared normal.
"We had never seen nuclei that looked like that," recalls Jaspersen. "It suggested that Mps3 was regulating the lipid environment of the nuclear envelope, and that perhaps that was how it controlled spindle pole body insertion," says Jaspersen.
To test their idea, the researchers expressed the MPS3-G186K mutant gene in a collection of yeast mutants, looking for ones that would fix the nuclear membrane defect. They found quite a few, and—as expected -- two had mutations in genes that regulate cellular lipid content. When they treated cells with oleic acid (essentially lard), or by slightly increasing the growth temperature -- presumably increasing membrane fluidity, they were able to suppress the defects.
"The nuclear envelope is not just a passive player but presumably is actively remodeled by Mps3 to accommodate the spindle pole body," explains Jaspersen.
Besides their prominent role in mitosis, centrosomes, the higher eukaryotic equivalent of yeast spindle pole bodies, are required for making primary cilia -- a hair-like appendage present in a single copy on every cell. At the base of primary cilia is a centrosome attached to the cell membrane that in cilia has a unique lipid composition. Jaspersen's group is anxious to see what aspects of their Mps3 findings translate to primary cilia. If parallels exist, the rewards could be significant, since ciliary defects can lead to a number of human diseases -- ranging from congenital heart failure to retinal degeneration.