Observations refute widely held view on causal mechanism in amyotrophic lateral sclerosis
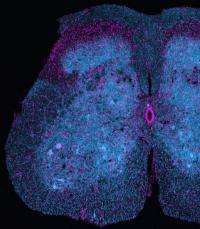
In science, refuting a hypothesis can be as significant as proving one, all the more so in research aimed at elucidating how diseases proceed with a view toward preventing, treating, or curing them. Such a discovery can save scientists from spending precious years of effort exploring a dead end. In a study published in the Proceedings of the National Academy of Sciences, Munich-based researchers refute a widely accepted hypothesis about a causative step in neurodegenerative conditions. These results deal specifically with animal models of human amyotrophic lateral sclerosis (ALS, aka Lou Gehrig's disease) but also raise questions for research on other neurodegenerative diseases, such as Alzheimer's or Huntington's disease.
One of the ways neurodegenerative diseases manifest themselves is in the loss of axons – essentially, the transmission lines for electrical signals in individual nerve cells – and synapses, the key sites for communication between them. In the past, such damage has been attributed to deficits in the bidirectional transport of organelles, such as the intracellular power plants called mitochondria, along the axons of nerve cells. Now, researchers at the Technische Universitaet Muenchen (TUM) and Ludwig-Maximilians-Universitaet Muenchen (LMU) have put that assumption under the microscope in the most thorough test to date. They used novel imaging techniques to observe changes in both axon morphology and organelle transport – with high resolution in both space and time – in several different animal models of ALS. Their results show that transport deficits and axon degeneration can develop independently, refuting the hypothesis that one is a direct cause of the other.
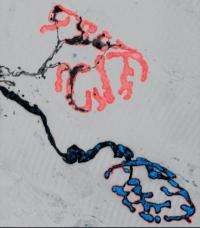
The lead researchers in this study were Prof. Thomas Misgeld of the TUM Institute of Neuroscience, a Fellow of the TUM Institute for Advanced Study, and Prof. Martin Kerschensteiner of the LMU Institute of Clinical Neuroimmunology. Together with Misgeld's research group, they observed axonal organelle transport in living tissue in real time – and in a way that enabled them to track the movement of individual mitochondria – using a novel imaging approach that involves transgenic labeling. They were also able to observe transport of another kind of organelle, endosome-derived vesicles. Several different animal models of ALS were investigated, all of which are based on human mutations associated with the disease.
"The methods – both in terms of labeling and in terms of live microscopy – that Martin Kerschensteiner and I have developed over the past years to track mitochondria in intact axons now allowed our student, Petar Marinković, to look directly into models of ALS," Misgeld explained. "The result came a bit as a surprise, as we fully expected a problem with transport of these organelles would be present in all the disease models and missing in all the controls. Well, the results taught us differently."
The researchers investigated a number of different questions arising from this surprising result, using multiple animal models of ALS and focusing on motor neurons, the kind of neuron most affected in this disease. By comparison, most previous studies of the putative link between organelle transport and ALS had been limited: Some, for example, focused on a single mutation, made their observations in vitro or in non-motor nerve cells, or did not include a wild-type mutation as a control; others measured surrogates for organelle transport deficits instead of observing transport directly.
Among the conclusions: In ALS models, reduction of organelle transport and initiation of axon degeneration appear to be due to different mechanisms. This suggests that, at least for ALS, axonal organelle transport may be an unsuitable therapeutic target.
In addition, Misgeld said, "We do think these insights have implications for other studies of ALS, or even studies of other neurodegenerative diseases. What our experiments really say is that it is not easy to develop faithful models of neurodegenerative diseases. So it might be worth spending more effort to get better animal models, as this is the only way forward for mechanistic studies, while always checking them against human pathology or human-derived cellular models. In the meantime, it is probably prudent to work with several of the available models in parallel. Moreover, in more general biological terms, our results also speak to the relationship between axonal transport disruptions and degeneration – which might not be as tight as we assumed. Here we have a lot more to understand."
More information: Axonal transport deficits and degeneration can evolve independently in mouse models of amyotrophic lateral sclerosis. Petar Marinković, Miriam S. Reuter, Monika S. Brill, Leanne Godinho, Martin Kerschensteiner, and Thomas Misgeld, PNAS Early Edition, Feb. 27-March 2, 2012. DOI: 10.1073/pnas.1200658109














