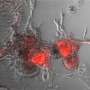
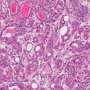
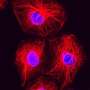
Inhibiting cell migration in breast cancer
(Medical Xpress) -- Scientists from the Friedrich Miescher Institute for Biomedical Research together with colleagues from the University of Fribourg have discovered a signaling mechanism controlling mobility and metastasis in breast cancer. They have been able to thus reduce invasiveness of the cancer cells. This is promising for the development of therapies against the types of breast cancer that readily form metastasis and for which a therapy has yet to be found.
Advances in the treatment of breast cancer in the last decades have decreased the mortality in breast cancer markedly. Despite these advances, one tough nut to crack remained: breast cancer that migrates early on into other tissues and forms metastases. For these aggressive tumors a therapy has yet to be found.
Scientists from the Friedrich Miescher Institute for Biomedical Research together with colleagues at the University of Fribourg have now discovered a handle on these aggressive tumors. The scientists in the groups of Brian A. Hemmings and Prof. Curzio Rüegg have been able to elucidate the signaling events that promote invasive behavior and metastasis in breast cancer. What is more, they could significantly impair the metastatic potential of these cells by a blockade of the signaling events.
In the breast cancer field, the transcription factor Twist1 has been known to play a role in motility and invasion in metastatic breast tumors. However the intracellular events regulating Twist1 have been poorly understood. The cancer scientist could now show that Twist1 exerts its devastating role through two signaling molecules: Akt and TGFß2. The hyper-activation of Akt, Twist1 and TGFß2 invariably leads to metastasis. It is a vicious cycle that the scientists discovered: Akt phosphorylates and thus activates Twist, which in turn leads to an increased production of TGFß2. And TGFß2 maintains the hyperactive Akt signaling through the TGFß receptor.
When the scientists depleted or inactivated one of the three molecules the cycle was broken, the cancer cells were less invasive and formed fewer metastases. "The results are promising; all the more that therapeutic entities inhibiting Akt are already in clinical development", comments Gongdua Xue, Postdoctoral Fellow in the group of Brian A. Hemmings and first author of the study. "In addition, we found that in 90% of the invasive breast tumors we tested Twist1 was continuously active. We believe thus that eventually a big fraction of the patients suffering from aggressive, invasive breast cancers could benefit from our findings."
More information: Xue G, et al. (2012) Akt/PKB-mediated phosphorylation of twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGFß signaling axes. Cancer Discovery, 2:193-195; doi:10.1158/2159-8290