ASCO: Continuing avastin with 2nd-line chemo ups survival
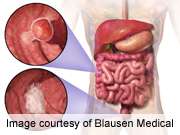
(HealthDay) -- Continuing use of bevacizumab (Avastin) in combination with second-line chemotherapy improves overall survival (OS) and progression-free survival (PFS) in patients with metastatic colorectal cancer (mCRC) who have progressed after discontinuation of first-line bevacizumab and chemotherapy, according to the results of a phase III study presented at the annual meeting of the American Society of Clinical Oncology, held from June 1 to 5 in Chicago.
Dirk Arnold, M.D., from the University Clinic Eppendorf in Hamburg, Germany, and colleagues randomized 820 patients with unresectable, histologically-confirmed mCRC, who had progressed within three months after discontinuation of first-line bevacizumab plus chemotherapy, to receive second-line fluoropyrimidine-based chemotherapy with bevacizumab (2.5 mg/kg/week equivalent; 409 patients) or without bevacizumab (411 patients). The choice of oxaliplatin- or irinotecan-based second-line chemotherapy was dependent on the regimen used in first-line treatment.
The researchers found that the median OS was significantly longer in those receiving bevacizumab and chemotherapy, compared to those receiving only chemotherapy (11.2 versus 9.8 months; hazard ratio [HR], 0.81), as was median PFS (5.7 versus 4.1 months; HR, 0.68). The adverse event profile was similar to previously reported data for bevacizumab and chemotherapy. When compared with historical data from bevacizumab treatment in first- and second-line mCRC treatment, bevacizumab-related adverse events were not increased by continuing treatment beyond progression.
"By simply switching chemotherapy drugs when the cancer progresses and continuing with bevacizumab, we can make second-line treatment even more powerful," Arnold said in a statement. "This finding will likely spur research into other cancer types that are sensitive to both bevacizumab and chemotherapy."
Several authors disclosed financial ties to pharmaceutical companies, including Genentech/Roche, the manufacturer of bevacizumab.
More information:
Abstract
More Information
Copyright © 2012 HealthDay. All rights reserved.