First major scientific study into rare inflammatory skin condition
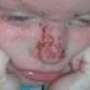
Pyoderma gangrenosum (PG) is a rare, disfiguring and very painful skin condition which affects around 360 people each year in the UK.
The condition results in large painful skin ulcers which take many months to heal — often with scarring. The condition is also associated with a higher risk of death. The cause of PG is not known, although patients often have underlying health conditions such as inflammatory bowel disease and rheumatoid arthritis and it is still not clear how best to treat PG when it does develop.
Now, for the first time dermatology experts led by Hywel Williams, Professor of Dermato-Epidemiology at The University of Nottingham, are carrying out the largest study of its kind in the world into this painful skin condition. The hope is that the results will help change clinical practice.
The randomised controlled trial is funded by the National Institute for Health Research (NIHR) as part of a Programme Grant for Applied Research awarded to the CEBD which is being managed by the Nottingham Clinical Trials Unit.
Professor Williams, Director of the Centre of Evidence Based Dermatology (CEBD), said: “Being told you have a rare skin condition and that very little evidence is out there to inform treatment because it is so uncommon is just not good enough in 2012. Many treatments have been tried, yet only one small clinical trial has ever been done on this condition. In order to guide doctors and patients about the best treatment, we have set up a national study together with University of Aberdeen to do a fair comparison of the two most commonly used treatments — oral steroids and ciclosporin.”
The search for answers
The STOP GAP Trial — Study of Treatments for Pyoderma Gangrenosum Patients — has already recruited just over a 175 patients — but more are needed before the study closes in October. It is hoped STOP GAP will provide some answers and inform clinicians on a “gold-standard” way of treating.
There has only ever been one other randomised controlled trial into the condition. This was carried out in 2006 and had a sample size of 30 patients. STOP GAP is recruiting patients from across the UK, in around 40 hospitals, and is comparing two commonly used oral treatments — prednisolone (a steroid) and ciclosporin (an immuno-supressant). Both medicines help PG to heal to some extent but it is not clear which is best and which is safest.
For more information about the trial please go to: www.stopgaptrial.co.uk