Do it like the immune system: novel antimicrobials
Microbial infections are becoming unbeatable due to progressive mutations that lead to antimicrobial drug resistance. European scientists exploited the characteristics of novel antimicrobial compounds that mimic the dual activities of natural antimicrobial proteins.
The fight against contagious diseases is seriously threatened by the growing development of antimicrobial-resistant pathogens. There is a profound need for novel and diverse antimicrobial prevention and therapeutic strategies.
Towards this direction, three pathways can be followed for the design of efficient drugs. The first is to target evolutionary conserved regions of the protein targets that have lower possibilities for change and therefore develop drug resistance. The second is the creation of drugs of multiple activities and targets leading to enhanced antimicrobial action and low resistance development. Finally, the third pathway involves drug cocktails of many active compounds of diverse activities and specificities.
The EU project 'Antimicrobials by immune stimulation' (AMIS) aimed at the design and evaluation of novel antimicrobial drugs that mimic features of human antimicrobial proteins. AMIS scientists combined all three aforementioned approaches in an integrative and innovative manner using the human innate system as a model for new antimicrobial compounds. Since in the human immune system antimicrobials comprise different signals in a single molecule, AMIS researchers made novel antimicrobial molecules of combined features.
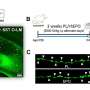
Initially, AMIS scientists collected and studied the properties of a plethora of promising natural antimicrobial molecules which have been identified in recent years. Taking the information from these studies, they designed antimicrobial proteins with dual activity. These proteins are able, on the one hand, to target extracellular or intracellular bacteria and, on the other, to weaken inflammation.
Within AMIS, important tools for the evaluation of antimicrobial proteins were optimised and validated. In addition, a variety of new compounds with either antimicrobial or anti-inflammatory activity were discovered.
Importantly, AIMS researchers managed to decipher the exact molecular mechanism of action in some of these compounds, essential for the design of effective drugs. Moreover, the most promising compounds were selected for the creation of fused compounds with even better characteristics. The first fused compound was created and is currently under evaluation.
The AMIS project did not result in a specific therapeutic strategy but it highlighted current limitations in the development of antimicrobial proteins against antimicrobial resistance. The reason for these limitations is not strictly technical but rather a lack of fundamental knowledge in the field of microbial pathogenesis and innate immune system.
More information: Information Source: Result from the EU funded FP6-LIFESCIHEALTH programme