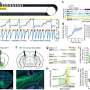
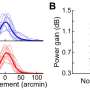
GEN reports on ocular therapeutics targeting the retina

Therapies for retinal diseases are expected to overtake those for glaucoma by 2014, reports Genetic Engineering & Biotechnology News (GEN). Because current retinal disease treatments only improve vision for six to eight weeks, there is a critical need for new remedies, according to a recent issue of GEN.
"As increasing numbers of baby-boomers continue to grow older, many will have to deal with eye diseases such as age-related macular degeneration," said John Sterling, Editor-in-Chief of GEN. "Some estimates put the current AMD and diabetic retinopathy drug segment of the market at $3 billion, and this is expected to increase to about $5 billion in two years."
Standard therapy has been Genentech's VEGF inhibitors Lucentis and the off-label use of Avastin®. Regeneron, in collaboration with Bayer HealthCare, is challenging these drugs with a similar VEGF inhibitor, Eyela. The FDA approved the drug last November for wet AMD.
In another approach, Acucela is in Phase II trials using visual cycle modulators to lighten the metabolic load on the retina by reducing the activity of the rod visual system. This protects the retina from light damage, improves retinal vasculature, and reduces the accumulation of A2E and other retinal-related toxic by-products.
GlaxoSmithKline has two drugs in Phase II trials for ocular therapy: darapladib, an oral Lp-PLA2 inhibitor for diabetic macular edema, and Votrient®, a multi-kinase angiogenesis inhibitor in eye drop form for AMD. Early-stage work also is under way for neovascular AMD, dry AMD, diabetic retinopathy, diabetic macula edema, uveitis, and glaucoma, as well as for technologies for drug delivery.