In treated MS, early disease activity predicts poor outcome
(HealthDay)—After 15 years of follow-up, patients with relapsing-remitting multiple sclerosis (RRMS) who display disease activity despite treatment with interferon (IFN)β-1a tend to have unfavorable long-term outcomes, according to research published online Sept. 13 in the Annals of Neurology.
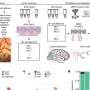
Robert A. Bermel, M.D., of the Cleveland Clinic, and colleagues conducted a multicenter, observational, 15-year follow-up study involving 136 patients with RRMS, who had initially been treated with either intramuscular (IM) IFNβ-1a or placebo, to identify early predictors of long-term outcomes.
The researchers found that significantly fewer patients treated with IM IFNβ-1a had early disease activity. Of those treated with IFNβ-1a, persistent disease activity was associated with an 8.96-fold higher risk of gadolinium-enhancing lesions, a 4.44-fold higher risk of relapse, and a 2.90-fold higher risk of new T2 lesions. Conversely, early disease activity in placebo-treated patients was not associated with long-term outcomes.
"Disease activity despite treatment with IFNβ is associated with unfavorable long-term outcomes. Particular attention should be paid to gadolinium-enhancing lesions on IFNβ therapy, as their presence strongly correlates with severe disability 15 years later," the authors write. "The results provide rationale for monitoring IFNβ treated patients with magnetic resonance imaging, and for changing therapy in patients with active disease."
This study was supported by Biogen Idec.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2012 HealthDay. All rights reserved.