2nd firm agrees to temporary shutdown in outbreak
(AP)—A Massachusetts company run by the same executives who operated a specialty pharmacy linked to a fatal meningitis outbreak has agreed to temporarily shut down for inspection by state and federal regulators.
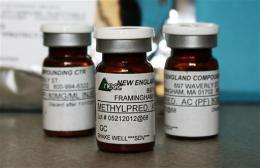
Ameridose is located in Westborough, Mass. The New England Compounding Center, which produced a steroid linked to the outbreak, is in Framingham. Both firms are run by Barry Cadden and Greg Conigliaro.
Ameridose provides sterile medication in prefilled oral syringes to about 3,000 hospitals nationwide. It opened its doors in 2006, eight years after NECC opened.
The Massachusetts Department of Public Health says Ameridose agreed to the shutdown until inspections by state regulators and the U.S. Food and Drug Administration are completed.
There is no recall of Ameridose products.
The outbreak has sickened 137 people in 10 states. Twelve have died.
Copyright 2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.