Breast cancer cells enticed to spread by 'tumorous environment' as well as genetic changes
(Medical Xpress)—A new study from Johns Hopkins researchers suggests that the lethal spread of breast cancer is as dependent on a tumor's protein-rich environment as on genetic changes inside tumor cells.
In a report in the Sept. 25 issue of the Proceedings of the National Academy of Sciences, the scientists conclude that a molecular signal in the protein meshwork surrounding the breast cancer cells may provide the critical trigger to initiate the life-threatening process of metastasis to distant sites in the body.
Moreover, their experiments suggest that the environment surrounding a tumor can even coax healthy breast cells to invade surrounding tissue just as cancer cells do, and that a healthy environment can cause cancer cells to stay put and not spread as they usually do.
"The most dangerous aspect of breast cancer is its ability to spread to distant sites, and most tumors are initially unable to do that," says Andrew Ewald, Ph.D., assistant professor of cell biology at the Johns Hopkins School of Medicine and member of the Institute for Basic Biomedical Sciences' Center for Cell Dynamics. Learning more specifically what triggers metastases may provide additional targets for preventing and treating the malignant process that causes cancer deaths, Ewald adds.
It's widely accepted that cancers acquire the ability to spread through the gradual accumulation of genetic changes, and experiments have also shown that these changes occur in parallel with changes in the protein content and 3-dimensional patterning of the protein meshwork that creates their immediate surroundings. What has been unclear is whether those immediate surroundings play a role in initiating and encouraging cancer's spread, or whether they are more "effect" then "cause."
To sort out the contributions of both the genetic changes and the environment, Ewald's team separated tumor cells from their surroundings by taking fragments of human breast tumors and embedding them in two different commercially available 3-D gels, one that mimics the protein meshwork surrounding healthy mammary tissue and another that mimics tumorous mammary tissue.
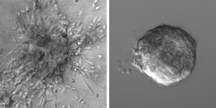
The gels are tools often used to study tumor invasiveness. The first was made of proteins that normally create a thin layer around healthy breast tissue, acting as a molecular boundary for it. The second was made entirely of a protein, collagen I, which is found in unusually high concentrations around breast tumors.
If cancer cells are driven to disperse solely because of the genetic changes they carry, the researchers expected to see the tumor fragments behave similarly in both the healthy and tumorous environments. What they saw instead, says Ewald, was a distinct difference. As expected, 88 percent of tumor fragments sent cells crawling into the tumorous meshwork environment, the first step in metastasis known as dissemination. (See video) But only 15 percent of tumor fragments sent cells crawling into the normal environment. (See video) According to Ewald, these results indicate that the environment around a tumor plays a more direct role in cancer spread than previously thought.
If indeed cells can be enticed outward by the protein environment, the researchers reasoned, that environment might even be powerful enough to coax cells away from healthy breast tissue. To test this idea, they took fragments of both healthy and cancerous mouse mammary glands and placed them in the collagen I gels mimicking a tumor's environment. Results show that in the tumorous environment, nearly as many of the healthy fragments sent cells dispersing (See video) as did the tumorous fragments (See video).
One notable difference shown in their time-lapse videos was that healthy cells only exited the normal tissue fragments for a short period of time, while cancer cells continued to exit the tumor fragments throughout the whole test period.
To learn why healthy cells stopped exiting normal tissue fragments sooner, the researchers analyzed the proteins on the cells that were in direct contact with the meshwork gels. In breast tissue, a thin protein layer normally forms a boundary between cells and their environment. When both healthy and tumorous tissue fragments were surgically removed prior to the experiments, this boundary was disrupted, allowing breast cells to directly contact the protein meshwork beyond the boundary. In healthy mammary gland fragments, the team found that the "self-corrective behavior" coincided with the re-creation of this protein boundary.
"This tells us that tumors continue to listen to their environments," Ewald says. "Our data suggest that tumors with genetic changes that favor cancer spread may not disperse until they are within a permissive environment." A dispersal-permissive environment is enough to provoke invasive behavior in all mammary tissue, healthy and cancerous, Ewald explains, and the difference between the two can be a single genetic change that allows the dispersal to continue unchecked.















