Stay-at-home transcription factor prevents neurodegeneration
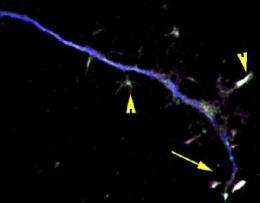
A study in The Journal of Cell Biology shows how a transcription factor called STAT3 remains in the axon of nerve cells to help prevent neurodegeneration. The findings could pave the way for future drug therapies to slow nerve damage in patients with neurodegenerative diseases.
In Lou Gehrig's Disease (ALS) and other neurodegenerative diseases, nerve cells usually die in stages, with axons deteriorating first and the cells themselves perishing later. Axon degeneration may represent a turning point for patients, after which so much nerve damage has accumulated that treatments won't work. Researchers have tested several proteins for their ability to save axons. One of these molecules, CNTF, rescues axons in rodents and extends their lives. But it caused severe side effects in patients during clinical trials. "Acting on the same pathway but farther downstream could be an ideal way to improve the situation for motor neuron disease" and possibly for other neurodegenerative diseases, says senior author Michael Sendtner from the University of Wuerzburg in Germany.
To discover how CNTF works, Sendtner and his colleagues studied mice with a mutation that mimics ALS. The researchers found that CNTF not only prevented shrinkage of the rodents' motor neurons, it also reduced the number of swellings along the axon that are markers of degeneration. It is known that CNTF indirectly turns on the transcription factor STAT3, so the researchers wanted to determine if STAT3 is behind CNTF's protective powers. They tested whether CNTF helps motor neurons that lack STAT3 and discovered that, in the mutant mice, axons lacking STAT3 were half as long as those from a control group after CNTF treatment
Once it has been activated, STAT3 typically travels to the nucleus of the neuron to switch on genes. But the researchers were surprised to find that most of the axonal STAT3 did not move to the nucleus and instead had a local effect in the axon. Specifically, the team found that activated STAT3 inhibited stathmin, a protein that normally destabilizes microtubules. When the team removed stathmin in motor neurons from the mutant mice, the axons grew at the same rate as axons from normal mice but didn't elongate any faster after doses of CNTF. These results indicate that CNTF mainly stimulates axon growth by thwarting stathmin and suggests that drugs to block stathmin could slow neuron breakdown in patients with neurodegenerative diseases.
More information: J. Cell Biol. doi:10.1083/jcb.201203109











.jpg)






