Combination of two pharmaceuticals proves effective in the treatment of multiple sclerosis

A new substance class for the treatment of multiple sclerosis and other neurodegenerative diseases now promises increased efficacy paired with fewer side effects.
To achieve this, a team of scientists under the leadership of Prof. Gunter Fischer (Max Planck Research Unit for Enzymology of Protein Folding, Halle/Saale, Germany) and Dr. Frank Striggow (German Center for Neurodegenerative Diseases (DZNE)) have combined two already approved pharmaceutical substances with each other using a chemical linker structure. The objectives of this combination are to ensure maximum brain cell protection on the one hand and the suppression of unwanted side effects on the other. The new class of substances has now been registered with the European Patent Office as the DZNE's first patent in the form of a joint patent application with the Max Planck Research Unit. "The patent approval process can take several years. During this phase we are planning to conclude the pre-clinical development. It is our aim to start with clinical research and development at the earliest possible time. Overall, we have identified substantial therapeutic potential as far as chronic and age-related neurodegenerative diseases are concerned," comments Dr. Frank Striggow.
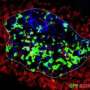
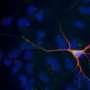
Multiple sclerosis is an inflammatory disease that affects the central nervous system. It destroys the insulation of the nerve cell signaling system, the myelin sheaths of the neural axons. The consequence of this process is the malfunction of signaling and finally cell death resulting in permanent neurological problems. The cause of multiple sclerosis is that the body itself attacks the cellular components of the myelin sheaths, the oligodendrocytes. Hence, the team of scientists under the direction of Prof. Gunter Fischer and Dr. Frank Striggow embarked on a search for intervention options that could protect brain cells from these attacks. The goal was not only to prevent the damage and loss of brain cells, but also to develop a medication that has a positive impact on cell regeneration.
The used components of the Cyclosporine and FK506 (tacrolimus)-series have been utilized in a chemically slightly altered form as immunosuppressant medications for a long time. Both suppress the cellular immune defenses. This effect is necessary in conjunction with organ transplants, but otherwise problematic for the organism. The specific combination of the two substances amplifies the protective effect on the nerve cells thanks to different but synergistic efficacy mechanisms. The impact on the immune defense is reduced at the same time, which results in fewer side effects. Both of these achievements were corroborated by experiments. An application for a patent protecting this new class of active ingredients has now been filed. Ascenion GmbH and Max Planck Innovation both attend the project as utilization partners of the DZNE and of the Max Planck Society.