It's not just what you eat, but when you eat it
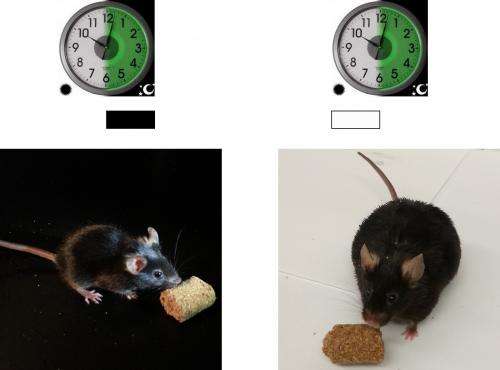
Fat cells store excess energy and signal these levels to the brain. In a new study this week in Nature Medicine, Georgios Paschos PhD, a research associate in the lab of Garret FitzGerald, MD, FRS director of the Institute for Translational Medicine and Therapeutics, Perelman School of Medicine, University of Pennsylvania, shows that deletion of the clock gene Arntl, also known as Bmal1, in fat cells, causes mice to become obese, with a shift in the timing of when this nocturnal species normally eats. These findings shed light on the complex causes of obesity in humans.
The Penn studies are surprising in two respects. "The first is that a relatively modest shift in food consumption into what is normally the rest period for mice can favor energy storage," says Paschos. "Our mice became obese without consuming more calories." Indeed, the Penn researchers could also cause obesity in normal mice by replicating the altered pattern of food consumption observed in mice with a broken clock in their fat cells.
This behavioral change in the mice is somewhat akin to night-eating syndrome in humans, also associated with obesity and originally described by Penn's Albert Stunkard in 1955.
The second surprising observation relates to the molecular clock itself. Traditionally, clocks in peripheral tissues are thought to follow the lead of the "master clock" in the SCN of the brain, a bit like members of an orchestra following a conductor. "While we have long known that peripheral clocks have some capacity for autonomy – the percussionist can bang the drum without instructions from the conductor – here we see that the orchestrated behavior of the percussionist can, itself, influence the conductor," explains FitzGerald.
Daily intake of food is driven by oscillating expression of genes that drive and suppress appetite in the hypothalamus. When the clock was broken in fat cells, the Penn investigators found that this hypothalamic rhythm was disrupted to favor food consumption at the time of inappropriate intake – daytime in mice, nighttime in humans.
When a species' typical daily rhythm is thrown off, changes in metabolism also happen. For example, in people, night shift workers have an increased prevalence of obesity and metabolic syndrome, and patients with sleep disorders have a higher risk for developing obesity. Also, less sleep means more weight gain in healthy men and women.
Balancing Act
Balancing energy levels in the body requires integrating mul¬tiple signals between the central nervous system and outlying tissues, such as the liver and heart. Fat cells not only store and release energy but also communicate with the brain about the amount of stored energy via the hormone leptin. When leptin is secreted, it causes more energy to be used and less eating via pathways in the hypothalamus.
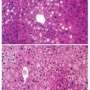
The Penn team found that only a handful of genes were altered when the clock was broken in fat cells and these governed how unsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were released into the blood stream. Interestingly, these are the same fatty acids that are typically associated with fish oils. Sure enough, levels of EPA and DHA were low in both plasma and in the hypothalamus at the time of inappropriate feeding. "To our amazement, we were able to rescue the entire phenotype - inappropriate fatty acid oscillation and gene expression in the hypothalamus, feeding pattern and obesity - by supplementing EPA and DHA to the knock-out animals," notes Paschos.
The findings point to a role for the fat cell clock molecules in organizing energy regulation and the timing of eating by communicating with the hypothalamus, which ultimately affects stored energy and body weight.
Taken together, these studies emphasize the importance of the molecular clock as an orchestrator of metabolism and reflect a cen¬tral role for fat cells in the integration of food intake and energy expenditure.
"Our findings show that short-term changes have an immediate effect on the rhythms of eating," says FitzGerald. "Over time, these changes lead to an increase in body weight. The conductor is indeed influenced by the percussionist."