Search for epigenetic decoder leads scientists to Rett Syndrome
(Medical Xpress)—A few years ago, scientists discovered an unexpected layer of information woven into the genetic code – a nucleotide called 5-hydroxymethylcytosine, or 5hmC. Its meaning was unknown at the time, but a new analysis suggests that a regulatory protein called MeCP2, known for its involvement in the nervous system disorder Rett Syndrome, recognizes 5hmC in the brain and facilitates activation of the genes in which it is most abundant.
The study, which includes the first maps of 5hmC's distribution throughout the genomes of three types of brain cells, indicates 5hmC has different effects on gene activity in the nervous system than it does in other cell types in which it has been studied. "This direct connection between 5hmC and Rett Syndrome will force two fast-moving and exciting fields to come together in a way that was totally unanticipated," says Howard Hughes Medical Institute investigator Nathaniel Heintz, who led the study. Heintz and his colleagues published their findings in the December 21, 2012, issue of the journal Cell.
A genome's instructions for building proteins are spelled out in its sequence of As, Ts, Cs, and Gs – the DNA building blocks more formally known as adenine, thymine, cytosine, and guanine. But just as important to shaping an organism's form and function are chemical modifications to those nucleotides that influence how the DNA code is read. These modifications, known as epigenetic changes, help control when and where genes are switched on.
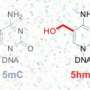
When Skirmantas Kriacionis in Heintz's lab at Rockefeller University discovered high levels of 5hmC – a modified form of cytosine that had previously been found only in bacterial viruses—in brain cells in 2009, the scientists immediately suspected the nucleotide was involved in epigenetic regulation. To figure out its significance, they began working to compile a map of where in the genome the new nucleotide could be found. 5hmC has since been found in other mammalian cells, but it is 10-20 times more abundant in the brain, so Heintz's team focused their study there.
Other researchers had noted that in embryonic stem cells, 5hmC tended to cluster around regulatory regions of the genome. In brain cells, however, the nucleotide was distributed across genes – suggesting that its effects on gene regulation might vary by tissue or even cell. Heintz, who has been working for decades to understand the differences between cell types in the nervous system (he estimates there are about 500), and his team chose three types of brain cells in which to map 5hmC: two types of neurons –Purkinje cells, which are large and elaborately branched, and granule cells, which are small and compact—as well as Bergmann glial cells, brain cells with an intermediary size and structure. Although all of these cell types contain the same genetic information, each activates a unique set of genes to establish its specialized structure and function.
Marian Mellen, a postdoctoral fellow in the lab, used a chemical label to pinpoint 5hmC in the DNA of each of the three types of cells. In addition, they mapped the locations of another modified version of cytosine, 5-methyl cytosine, or 5mC. 5mC is a well studied epigenetic modification known to silence genes. Their maps revealed that in each cell type, 5hmC was most abundant in active genes. Their data also showed that genes with high levels of 5hmC had low levels of the gene-silencing 5mC – though the strength of this relationship depended on the cell type.
"If you had to state a general rule, it would probably be that the higher the ratio of 5hmC to 5mC in a gene body in the nervous system, the more likely the gene will be expressed at high levels," Heintz summarizes. "But transcriptional control is cell-specific." Even in the three cell types they have so far studied, he says, the strength of each nucleotide's effects on gene activity are not equivalent.
To search for the proteins that decode the information communicated by 5hmC in the brain, Kriaucionis and graduate student Pinar Ayata searched for proteins in brain cells that stuck to beads that they had coated with 5hmC. They found only one such protein, and were able to identify it as the regulatory protein known as methyl C-p-G binding protein 2 (MeCP2).
"MeCP2 is present at very high levels in the nervous system and at vanishingly low levels in the periphery—so maybe it's not surprising that this epigenetic mark that is largely nervous system specific is recognized by a protein that is also nervous system specific," Heintz says. "But to find that this well known, well studied protein is directly connected to hydroxymethylcytosine was really shocking."
MeCP2 is best known for its involvement in Rett Syndrome. In children with Rett Syndrome, a mutation in the protein causes language and growth retardation, breathing problems, seizures, motor dysfunction, hand-wringing, and social impairment. In 2008, HHMI investigator Huda Zoghbi, who discovered MeCP2's link to Rett Syndrome, showed that in certain parts of the brain, the mutated protein alters the expression of about 2,500 genes. MeCP2 is now known to help inactivate genes marked by 5mC.
The new findings suggest MeCP2 can also help activate genes in which the concentration of 5hmC is high. In fact, experiments done by Ayata indicate that the regulatory protein has an equal affinity for binding to either of the two nucleotides. Heintz plans to investigate how the same protein can trigger these opposing effects, depending on which nucleotide it binds.
"This is nice situation where trying to study the biology of a particular cell type led us to an area of investigation that we had not anticipated," he says, noting that he is eager to explore 5hmC's involvement in Rett Syndrome. "It's rare that you get a chance to be thrust into a field that you really didn't think you were going to be inhabiting—but if you follow your experiments and believe what they tell you, you can end up in very different places than you had thought."