To revert breast cancer cells, give them the squeeze
Researchers at the University of California, Berkeley, and the Lawrence Berkeley National Laboratory have put the squeeze—literally—on malignant mammary cells to guide them back into a normal growth pattern.
The findings, to be presented Monday, Dec. 17, at the annual meeting of the American Society for Cell Biology in San Francisco, show for the first time that mechanical forces alone can revert and stop the out-of-control growth of cancer cells. This change happens even though the genetic mutations responsible for malignancy remain, setting up a nature-versus-nurture battle in determining a cell's fate.
"We are showing that tissue organization is sensitive to mechanical inputs from the environment at the beginning stages of growth and development," said principal investigator Daniel Fletcher, professor of bioengineering at UC Berkeley and faculty scientist at the Berkeley Lab. "An early signal, in the form of compression, appears to get these malignant cells back on the right track."
Throughout a woman's life, breast tissue grows, shrinks and shifts in a highly organized way in response to changes in her reproductive cycle. For instance, when forming acini, the berry-shaped structures that secrete milk during lactation, healthy breast cells will rotate as they form an organized structure. And, importantly, the cells stop growing when they are supposed to.
One of the early hallmarks of breast cancer is the breakdown of this normal growth pattern. Not only do cancer cells continue to grow irregularly when they shouldn't, recent studies have shown that they do not rotate coherently when forming acini.
While the traditional view of cancer development focuses on the genetic mutations within the cell, Mina Bissell, Distinguished Scientist at the Berkeley Lab, conducted pioneering experiments that showed that a malignant cell is not doomed to become a tumor, but that its fate is dependent on its interaction with the surrounding microenvironment. Her experiments showed that manipulation of this environment, through the introduction of biochemical inhibitors, could tame mutated mammary cells into behaving normally.
The latest work from Fletcher's lab, in collaboration with Bissell's lab, takes a major step forward by introducing the concept of mechanical rather than chemical influences on cancer cell growth. Gautham Venugopalan, a member of Fletcher's lab, conducted the new experiments as part of his recently completed Ph.D. dissertation at UC Berkeley.
"People have known for centuries that physical force can influence our bodies," said Venugopalan. "When we lift weights, our muscles get bigger. The force of gravity is essential to keeping our bones strong. Here we show that physical force can play a role in the growth—and reversion—of cancer cells."
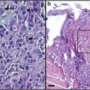
Venugopalan and collaborators grew malignant breast epithelial cells in a gelatin-like substance that had been injected into flexible silicone chambers. The flexible chambers allowed the researchers to apply a compressive force in the first stages of cell development.
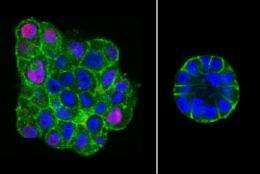
Over time, the compressed malignant cells grew into more organized, healthy-looking acini that resembled normal structures, compared with malignant cells that were not compressed. The researchers used time-lapse microscopy over several days to show that early compression also induced coherent rotation in the malignant cells, a characteristic feature of normal development.
Notably, those cells stopped growing once the breast tissue structure was formed, even though the compressive force had been removed.
"Malignant cells have not completely forgotten how to be healthy; they just need the right cues to guide them back into a healthy growth pattern," said Venugopalan.
Researchers further added a drug that blocked E-cadherin, a protein that helps cells adhere to their neighbors. When they did this, the malignant cells returned to their disorganized, cancerous appearance, negating the effects of compression and demonstrating the importance of cell-to-cell communication in organized structure formation.
It should be noted that the researchers are not proposing the development of compression bras as a treatment for breast cancer. "Compression, in and of itself, is not likely to be a therapy," said Fletcher. "But this does give us new clues to track down the molecules and structures that could eventually be targeted for therapies."
More information: "Externally applied forces can phenotypically revert malignant breast epithelial structures," Monday, Dec. 17, 2012, 2−3:30 pm, Session: Tumor Microenvironment, presentation: 1673, poster: B1444, Exhibit Halls A-C