Researchers find new clue to clinical trial failures of MMP cancer therapies

Proposed cancer therapeutic drugs based on blocking the catalytic activities of matrix metalloproteinases (MMPs), which profoundly remodel the environment surrounding a breast cell, have performed poorly in clinical trials. In mouse studies of MMP14, an enzyme that is often highly expressed in breast cancer, Berkeley Lab researchers have found a possible clue as to why. If confirmed for other MMPs, the finding could point the way to new strategies for future MMP-based cancer therapies.
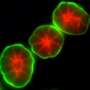
Berkeley Lab Distinguished Scientist Mina Bissell led a study of MMP14 in nulliparous mice that shows it does not promote invasive behavior in the mammary gland through its catalytic domain, as has been almost universally believed by the cancer research community. Instead, MMP14 promotes invasion through its transmembrane domain, specifically through the direct association of that domain with the beta 1 subunit of integrin (Itgb1), a signaling molecule involved in mammary gland branching and invasion into the fat pad. The Bissell lab previously showed that mis-regulation of Itgb1 plays a role in breast cancer.
"The reason anti-MMP drugs failed in clinical trials may be that they were aimed at the catalytic domain of these enzymes, which we all thought was the culprit," Bissell says. "The assumption has been that all MMP functions are in the catalytic domain, but we found that MMP14 promotes cancer through its transmembrane domain by mediating signals from the extracellular matrix (ECM) via cross talk and association with Itgb1."
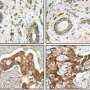
Since high expression of MMP14 has been strongly linked to poor outcomes, including increased death, for breast cancer patients, this membrane-bound enzyme became a prime target for pharmaceutical companies seeking to develop breast cancer therapies. The idea was to inhibit not only MMP14, but also any of the other MMPs from functioning.
"The failure and indeed resulting damage of all anti-MMP drugs in clinical trials indicated that MMPs as a class have useful functions that were also being blocked, leading to bad consequences for patients" Bissell says.
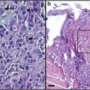
The essential non-proteolytic MMP14 activity that Hidetoshi Mori, a scientist in Bissell's research group lab, and other collaborators identified was the association between the transmembrane/cytoplasmic domain of MMP14 and Itgb1 molecules. The MMP14 was found to be expressed in the mammary epithelial cells of nulliparous mice at the tips of mammary gland branches, where it binds with Itgb1 to control the intracellular and extracellular signals that enable the cells to navigate and invade the fatty stroma.
"Invasion of the stroma and branching by mammary epithelial cells are required for normal breast development. Silencing the MMP14 prevents epithelial cells from moving into the stroma and reduces Itgb1 levels, which results in less branching," Mori says.
Adds Bissell, "When MMP14 interacts with Itgb1 in a tumor, however, these same processes are subverted, the tumor is able to hijack normal cell pathways and cancer is promoted."
These findings suggest that the non-catalytic activity in MMPs could provide a new target for future clinical strategies.