Expert questions US public health agency advice on influenza vaccines
The United States government public health agency, the CDC, pledges "To base all public health decisions on the highest quality scientific data, openly and objectively derived." But Peter Doshi, a postdoctoral fellow at Johns Hopkins University School of Medicine, argues that in the case of influenza vaccinations and their marketing, this is not so. His article is published on BMJ website today
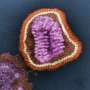
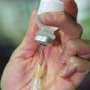
Promotion of influenza vaccines is one of the most visible and aggressive public health policies today, writes Doshi. Today around 135 million doses of influenza vaccine annually enter the US market, with vaccinations administered in drug stores, supermarkets - even some drive-throughs.
This enormous growth has not been fuelled by popular demand but instead by a public health campaign that delivers a straightforward message: influenza is a serious disease, we are all at risk of complications from influenza, the flu shot is virtually risk free, and vaccination saves lives.
Yet, Doshi argues that the vaccine might be less beneficial and less safe than has been claimed, and the threat of influenza appears overstated.
To support its case, the CDC cites two studies of influenza vaccines, published in high-impact, peer-reviewed journals and carried out by academic and government researchers with non-commercial funding. Both found a large (up to 48%) relative reduction in the risk of death.
"If true, these statistics indicate that influenza vaccines can save more lives than any other single licensed medicine on the planet," says Doshi. But he argues that these studies are "simply implausible" and likely the product of the 'healthy-user effect' (in this case, a propensity for healthier people to be more likely to get vaccinated than less healthy people).
In addition, he says, there is virtually no evidence that influenza vaccines reduce elderly deaths – the very reason the policy was originally created.
He points out that the agency itself acknowledges the evidence may be undermined by bias. Yet, he says "for most people, and possibly most doctors, officials need only claim that vaccines save lives, and it is assumed there must be solid research behind it."
He also questions the CDC's recommendation that beyond those for whom the vaccine is contraindicated, influenza vaccine can only do good, pointing to serious reactions to influenza vaccines in Australia (febrile convulsions in young children) and Sweden and Finland (a spike in cases of narcolepsy among adolescents).
Doshi suggests that influenza is yet one more case of "disease mongering" - medicalising ordinary life to expand markets for new products. But, he warns that unlike most stories of selling sickness, "here the salesmen are public health officials, worried little about which brand of vaccine you get so long as they can convince you to take influenza seriously."
But perhaps the cleverest aspect of the influenza marketing strategy surrounds the claim that "flu" and "influenza" are the same, he concludes. "All influenza is "flu," but only one in six "flus" might be influenza. It's no wonder so many people feel that "flu shots" don't work: for most flus, they can't."
Earlier this year, the BMJ launched a 'Too Much Medicine' campaign to help tackle the threat to health and the waste of money caused by unnecessary care. The journal will also partner at an international conference Preventing Overdiagnosis to be held in September in the USA.
More information: www.bmj.com/cgi/doi/10.1136/bmj.f3037