Cancer biology: Targeting tumors with 'stapled' peptides
(Phys.org) —Designer peptides containing chemically stabilized helices emerge as a potent way to activate anti-tumor proteins inside cells.
Cancer biologists consider many cancer-related proteins as 'undruggable' because they resist treatments from traditional drugs. David Lane and co-workers at the A*STAR p53 Laboratory, Bioinformatics Institute and Experimental Therapeutics Centre are therefore developing a new generation of therapeutics that can reach such proteins and activate their innate cancer-fighting abilities. The team's latest work reveals that 'stapled' peptides—chemically stabilized helices of amino acids—can activate the tumor-suppressing protein p53 inside cells by disrupting interactions with Mdm2, its regulator protein1.
Chemical biologists have realized that interfering with the crucial p53:Mdm2 interaction is a viable cancer treatment strategy; however, it is no easy task to find drug compounds that permeate cells and survive to reach their targets. Peptide ?-helices that adhere tightly to Mdm2's p53 binding domain make attractive drug candidates because of their low toxicity and site-specific potency. Unfortunately, native peptide helices tend to unravel and decompose inside cells.
To remedy this, Lane and co-workers explored the concept of holding peptide ?-helices in place by connecting parts of them with a hydrocarbon chain. First, they studied potential peptides using phage display, a technology that enables precise expression and rapid screening of peptide chains on the surfaces of virus-like particles.
By combining computer modeling with in vitro testing, the team generated stapled peptides known as 'sMTIDEs' with extraordinary affinity for binding p53. "Peptides isolated from phage display experiments are highly specific and potent against the individual protein they are selected against," says Christopher Brown, the study's lead author.
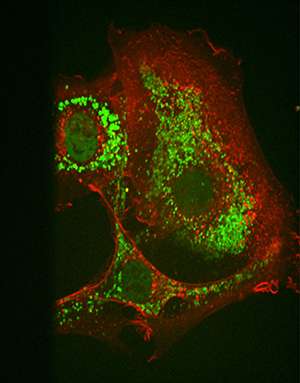
The researchers observed some surprising results when they compared the biological activity of their sMTIDE peptides to SAH-8, a stapled peptide derived from wild-type p53, and Nutlin, a small molecule that affects p53 production. The sMTIDEs could activate the p53 genes inside cell reporter assays quickly and at low concentrations. Their stapled peptide reached unprecedented levels of p53 activation and proved to have few toxic side-effects (see image). Furthermore, by making a single amino acid substitution on the sMTIDE chain, the researchers could activate or extinguish this activity without affecting in vitro binding.
Brown believes that these findings could help resolve a controversy brewing among scientists over the intracellular activity of stapled peptides. Peptides isolated from wild-type proteins, he notes, may show off-target binding because of evolved interactions with other proteins. The sMTIDEs, on the other hand, "prove the claims that peptides can enter cells. This has re-invigorated interest in the field and gives peptide researchers a set of validated and robust reagents," he says.
















