Modeling of congenital amegakaryocytic thrombocytopenia using iPS cell technology
A research group led by researcher Shinji Hirata and Professor Koji Eto at CiRA has conducted a study in which iPS cells generated from a patient with congenital amegakaryocytic thrombocytopenia (CAMT) were induced to differentiate into blood cells in vitro and then used to undertake a detailed study of the differences between these and cells from healthy subjects. The researchers found that, in humans, thrombopoietin receptors are essential not only to the maintenance of the multipotent hematopoietic progenitor population and the production of platelets, but also to erythropoiesis (red blood cell production).
CAMT is caused by the congenital loss of thrombopoietin receptor-mediated intracellular signaling, resulting in a state of severe thrombocytopenia from birth and leading in the infant period to bone marrow failure and gradual depletion first of red blood cells and then of white blood cells. This grave illness requires bone marrow transplant for the patient to survive, but the lack of an adequate experimental model has hitherto been a hindrance to elucidating its pathology. In the present study, with the cooperation of one CAMT patient who has now received a bone marrow transplant and recovered, iPS cells (CAMT iPS cells) were generated and induced in vitro to differentiate into blood cells, whose behavior was analyzed in detail.
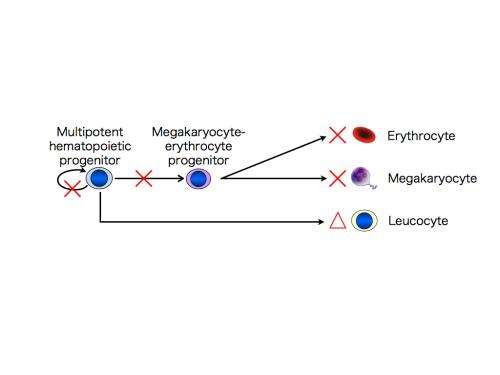
In the CAMT iPS cells, as in the CAMT patient's original blood cells, the thrombopoietin receptors were not functional. To check their ability to differentiate into megakaryocytes, platelets, erythrocytes (red blood cells), and other cell types, these iPS cells were differentiated into multipotent hematopoietic progenitors, which can in turn differentiate into a range of different blood cell types. It was found that the multipotent hematopoietic progenitors derived from the CAMT iPS cells, unlike the iPS cells from healthy subjects, had impaired ability to develop not only into megakaryocytes and platelets, but also into erythrocytes. They did however retain some ability to differentiate into white blood cells. These characteristics closely reflect the pathological state seen in CAMT patients (Fig. 1).
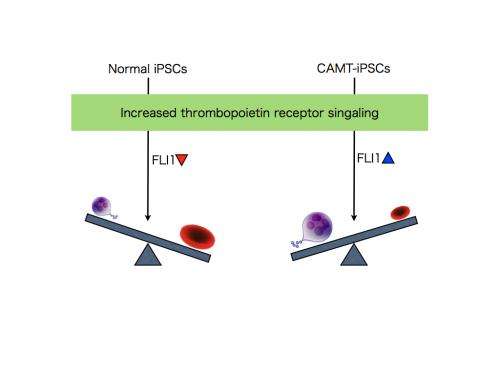
Next, the CAMT iPS cells were subjected to compensatory transduction of thrombopoietin receptors via genetic engineering, which normalized the production of all three of megakaryocytes, platelets, and erythrocytes. Altering the amount of thrombopoietin receptor introduced to bring it to the same level as in healthy subjects produced a differentiation pattern closely resembling that of healthy subjects, with a tendency to more erythrocytes than megakaryocytes. Intriguingly, overexpression promoted differentiation into megakaryocytes over differentiation into erythrocytes by CAMT patient-derived subjects. To investigate the fate-determining mechanism that decides between differentiation into megakaryocytes and erythrocytes, the researchers examined the transcription factors that operate downstream of thrombopoietin receptor signaling. This examination showed that, when thrombopoietin receptor signaling was intensified during the process of differentiation into megakaryocytes and erythrocytes, FLI1 was reduced in healthy subjects, but was in contrast elevated in CAMT iPS cells (Fig. 2).
By using iPS cells generated from CAMT patients, the research team succeeded in reproducing the pathological state of CAMT, in which the production of megakaryocytes and erythrocytes is markedly decreased compared to white blood cell production. Additionally, the team established that, in humans, thrombopoietin receptor signaling plays an important role in the maintenance of multipotent hematopoietic progenitors and their differentiation into megakaryocyte-erythrocyte progenitors, and is therefore essential to erythropoiesis. This is a finding which had not been possible with the mouse model that is in general use worldwide. In this way, using iPS cell technology has made it possible to not only analyze disease states, but also to investigate the mechanism of hematopoiesis. The findings of the study suggest that the thrombopoietin-like drugs used up till now to boost platelet count may also be useful in treating anemia. Going forward, iPS cells that model this disease will serve as an important tool in researching the origins of human hematopoiesis and the hematopoiesis pathway.















