New insights into control of neuronal circuitry could lead to treatments for an inherited motor disorder
The cerebellum is a region of the brain critical for balance, learning of motor skills and coordination of movements. In the outer layer of the cerebellum, individual 'Purkinje' cells integrate inputs from the brain stem and hundreds of thousands of granule cells to produce the cerebellar 'output'. Maintenance of the connections between Purkinje cells and associated parallel fibers is critical for proper cerebellar function, but very little is known about the underlying molecular mechanisms.
A team of researchers led by Katsuhiko Mikoshiba from the RIKEN Brain Science Institute in Wako has now identified a signaling molecule responsible for maintaining the integrity of these neuronal circuits in the mature cerebellum.
The type 1 inositol trisphosphate receptor (IP3R1) is known to be expressed at high levels in Purkinje cells. Mutations in the IP3R1 gene lead to uncoordinated movements, abnormal Purkinje cell structure and impaired signaling between Purkinje cells and parallel fibers in mice, and cause a human disease called spinocerebellar ataxia 15 (SCA15). Mikoshiba and his colleagues investigated the role of IP3R1 in the mature cerebellum by genetically engineering mice specifically lacking the receptor in their Purkinje cells.
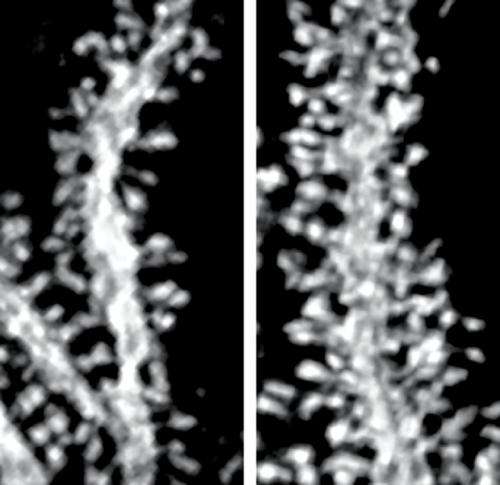
The researchers found that the mutant mice displayed impaired motor skill learning and severely uncoordinated movements, or ataxia, as seen in patients with SCA15. Closer examination of the cerebellum under the microscope also revealed abnormalities in the mice's Purkinje cells. While appearing to develop normally, in the adult animals these cells showed a dramatic increase in the density and length of their dendritic spines—the tiny finger-like protuberances that form connections with other cells (Fig. 1). All of the spines, however, formed fully functional connections with parallel fibers in the adult animals.
Previously, Mikoshiba's group showed that IP3R1 plays a critical role in a process called synaptic plasticity, by which connections between neurons are strengthened or weakened during learning. These new findings show that the receptor is also required for maintaining the proper spatial arrangement of connections in the adult cerebellum.
"Mice lacking IP3R1 specifically in Purkinje cells display ataxia similar to SCA15 patients," says Mikoshiba. He notes that since the abnormal maintenance of Purkinje cell dendritic spines appears to be associated with severe ataxia in the mutant mice, defects in the maintenance of the cerebellar circuit might similarly be involved in SCA15 pathogenesis.
"We are now studying the precise mechanism of how IP3R1 regulates Purkinje cell spine maintenance. This may elucidate SCA15 pathogenesis and lead to the development of new therapies," adds Mikoshiba.
More information: Sugawara, T., et al.Type 1 inositol trisphosphate receptor regulates cerebellar circuits by maintaining the spine morphology of Purkinje cells in adult mice, The Journal of Neuroscience 33, 12186–12196 (2013).dx.doi.org/10.1523/JNEUROSCI.0545-13.2013