New technology may boost bone growth response for spinal fusion

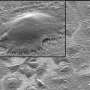
(Medical Xpress)—A spinal interbody fusion implant with a roughened titanium alloy surface provides an enhanced environment for bone formation, implant stability and fusion compared to one with a smooth titanium alloy surface, according to a new preclinical study led by the Virginia Commonwealth University School of Engineering.
Spinal fusion may be a necessary surgery for patients with broken vertebrae, deformities of the spine, certain spinal disorders, herniated disks or chronic low back pain to permanently connect two or more vertebrae in the spine to eliminate motion between them.
In order for spinal fusion to occur, an environment conducive to supporting bone formation and remodeling must be created. This is done through the use of spinal interbody fusion implants with specialized surfaces that are used to promote growth of bone and formation of blood vessels to provide nutrients and sustained bone health. Past research has focused mainly on bone growth factors and has overlooked blood vessel factors.
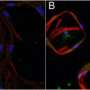
In the study, published online in The Spine Journal, researchers compared the production of cellular growth factors involved with both bone and blood vessel formation by bone cells cultured on two different environments – a smooth titanium alloy surface as well as a material commonly used for spine implants, poly-ether-ether-ketone, or PEEK. In addition, cells were cultured on a new proprietary implant surface technology created by medical device company, Titan Spine, LLC.
Through in vitro studies, the team demonstrated that rough titanium alloy surfaces increased production of the necessary growth factors by nearly 100 percent, compared to the PEEK and smooth titanium alloy materials.
"This means that by modifying titanium alloy surfaces to stimulate bone cells to produce these important factors, surgeons may be able to improve the performance of spine cages and, as a result, quality of care for their patients," said Barbara D. Boyan, Ph.D., dean of the VCU School of Engineering, who led the study.
According to Boyan, future work will move the team to better understand how surface design can impact the inflammation associated with spine implant surgery to further improve implant performance.
The study is titled: "Rough titanium alloys regulate osteoblast production of angiogenic factors."