Antibody fragment ameliorates first hallmarks of Alzheimer's disease in mice
Researchers at the Biosciences Unit of the Department of Biochemistry and Molecular Biology at the Universitat Autònoma de Barcelona (UAB), in collaboration with the UAB Institute of Neurosciences (INc), have conducted trials with mice by injecting a specific antibody fragment against soluble aggregates of the Ab peptide, responsible for the toxicity and cell death characteristic of Alzheimer's disease. The beneficial effects were seen at the behavioural, cellular and molecular levels five days after an intraperitoneal dose was administered.
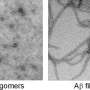
Since the first case of Alzheimer's disease was described, the disease has been associated with the presence of insoluble deposits known as amyloid plaques. However, in the past decade researchers have been able to conclude that the neuronal death characteristic of the disease is not due to the presence of these plaques but rather to the toxicity of the soluble aggregates preceding them (and called oligomers), formed by the Ab peptide.
Immunotherapy, consisting of the use of antibodies as a treatment for disease, is turning out to be an encouraging tool for the treatment of certain types of cancer and has also been used in clinical trials to treat Alzheimer's disease. Nevertheless, the clinical trial which had advanced the most in treating Alzheimer's through passive vaccination - using the bapineuzumab antibody - was halted in 2012 during its last trial phase due to controversial adverse effects and benefits of the treatment. Many scientists think the effects were the result of administering complete antibodies, which produce inflammation in the brain. For this reason, they propose that administering antibody fragments instead would be much safer.
The research group directed by Dr Sandra Villegas, from the Biosciences Unit of the Department of Biochemistry and Molecular Biology at the UAB, designed a recombinant antibody fragment (the single-chain variable fragment scFv-h3D6), a derivative of bapineuzumab, which only consists of the active part trapping the etiological agent of the disease: the domains of the antibody responsible for the binding of Aβ oligomers. Scientists observed how this antibody fragment protected from cell death in human cell-cultures and described the molecular mechanism by which this antibody fragment removed the Aβ oligomers that cause the disease.
In the latest edition of mAbs (monoclonal antibodies), a journal specialized in immunotherapy, the research group has published three articles which demonstrate the benefits of the treatment using the antibody fragment scFv-h3D6 in mice, and have redesigned the molecule to make it even more efficient.
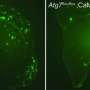
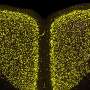
The mice come from the 3xTg-AD colony, an animal model of Alzheimer's disease, provided by Dr Lydia Giménez-Llort of the UAB Institute of Neurosciences (INCs). Researchers observed how a single injection into the abdomen of the animals and five days later, the mice reversed their levels of anxiety to normal levels and the learning and memory deficits were ameliorated. At the molecular level, researchers demonstrated two important facts: first, the treatment cleared from the cerebral cortex the Ab peptide oligomers, the elements causing the disease; and second, this clearence is linked to the recovery of the levels of certain apolipoproteins suspected to be the natural removers of Aβ peptide aggregates. The study on these apolipoproteins was conducted in collaboration with Dr Jose L. Sánchez-Quesada, from the Research Institute of the Sant Pau Hospital.
The results of the studies at the cellular level were also very encouraging. In addition to demonstrating that in young mice with the disease neuronal death occurs even in the cerebellum, UAB scientists observed how the antibody fragment protected the neurons, either fully in the less involved areas or partially in the most involved ones.
With the goal of improving the molecule, especially in regard to how long it can remain in the blood stream, the UAB group redesigned the molecule based on a molecular model developed in collaboration with Dr Baldo Oliva from the UPF-IMIM. The mutations introduced increase the thermodynamic stability by 25% and decrease the tendency to aggregate to some 4ºC, traits which clearly increase the therapeutic potential of the scFv-h3D6 fragment to treat Alzheimer's disease. Additionally, the published redesign can also be useful for other antibody fragments being produced in other laboratories with the aim of finding effective treatments for several diseases.
More information: Gimenez-Llort, L. et al. Early intervention in the 3xTg-AD mice with an amyloid β-antibody fragment ameliorates first hallmarks of Alzheimer disease, mAbs, 5:5, 665–677; September/October 2013.
Esquerda-Canals, G. et al. Loss of deep cerebellar nuclei neurons in the 3xTg-AD mice and protection by an anti-amyloid β antibody fragment, mAbs 5:5, 660–664; September/October 2013.
Rivera-Hernandez, G. et al. Elongation of the C-terminal domain of an anti-amyloid β single-chain variable fragment increases its thermodynamic stability and decreases its aggregation tendency, mAbs 5:5, 678–689; September/October 2013.