Critical gene in retinal development and motion sensing identified (w/ Video)
Our vision depends on exquisitely organized layers of cells within the eye's retina, each with a distinct role in perception. Johns Hopkins researchers say they have taken an important step toward understanding how those cells are organized to produce what the brain "sees." Specifically, they report identification of a gene that guides the separation of two types of motion-sensing cells, offering insight into how cellular layering develops in the retina, with possible implications for the brain's cerebral cortex. A report on the discovery is published in the Nov. 1 issue of the journal Science.
"The separation of different types of cells into layers is critical to their ability to form the precise sets of connections with each other—the circuitry—that lets us process visual information," says Alex Kolodkin, Ph.D., a professor in the Johns Hopkins University School of Medicine's Solomon H. Snyder Department of Neuroscience and an investigator at the Howard Hughes Medical Institute. "There is still much to learn about how that separation happens during development, but we've identified for the first time proteins that enable two very similar types of cells to segregate into their own distinct neuronal layers."
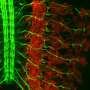
Kolodkin's research group specializes in studying how circuitry forms among neurons (brain and nerve cells). Past experiments revealed that two types of proteins, called semaphorins and plexins, help guide this process. In the current study, Lu Sun, a graduate student in Kolodkin's laboratory, focused on the genes that carry the blueprint for these proteins in two of the 10 layers of cells in the mammalian retina.
Those two layers are made up of so-called starburst amacrine cells (SACs). One type of SAC, known as "Off," detects motion by sensing decreases in the amount of light hitting the retina, while the other type, "On," detects increases in light. Sun examined the amounts of several semaphorin and plexin proteins being made by each type of cell, and found that only the "On" SACs were making a semaphorin called Sema6A. Sema6A can only work in the retina by interacting with its receptor, a plexin called PlexA2, but Sun found both types of SAC were churning out roughly equal amounts of PlexA2.
Reasoning that Sema6A might be the key difference that enabled the "On" and "Off" SACs to segregate from one another, Kolodkin's team analyzed mice in which the genes for either Sema6A, PlexA2 or both could be switched off, and looked at the effects of this manipulation on their retinas. "Knocking out" either gene during development led the "On" and "Off" layers to run together, the team found, and caused abnormalities in the "On" SACs' tree-like extensions. However, the "Off" SACs, which hadn't been using their Sema6A gene in the first place, still looked and functioned normally.
"When signaling between Sema6A and PlexA2 was lost, not only was layering compromised, but the 'On' SACs lost both their distinctive symmetrical appearance, and, importantly, their motion-detecting ability," Sun says. "This is evidence that the beautiful symmetric shape that gives starburst amacrine cells their name is necessary for their function."
Adds Kolodkin, "We hope that learning how layering occurs in these very specific cell types will help us begin sorting out how connections are made not just in the retina, but also in neurons throughout the nervous system. Layering also occurs in the cerebral cortex, for example, which is responsible for thought and consciousness, and we really want to know how this is organized during neural development."
More information: "On and Off Retinal Circuit Assembly by Divergent Molecular Mechanisms," by L.O. Sun et al. Science, 2013.