Elucidation of mechanism underlying acquisition of specificity for ATP
The mechanism underlying the acquisition of specificity for ATP has been elucidated by researchers in a new report published in Scientific Reports on September 11th, 2013.
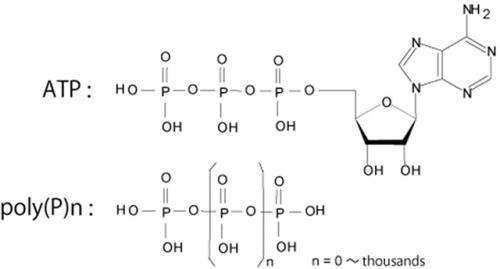
Inorganic polyphosphate [poly(P)], a polymer of orthophosphate (Pi) residues linked by high-energy phosphoanhydride bonds, is found in every organism from bacteria to animals (Fig.1). ATP is a universal energy carrier, sometimes termed the "energy currency" of cells (Fig.1). NAD kinase (NADK) catalyzes the phosphorylation of NAD+ to yield NADP+, using poly(P) or ATP as a source of phosphate and energy. poly(P)/ATP-NADK utilizes both poly(P) and ATP, whereas ATP-specific NADK prefers ATP to poly(P).
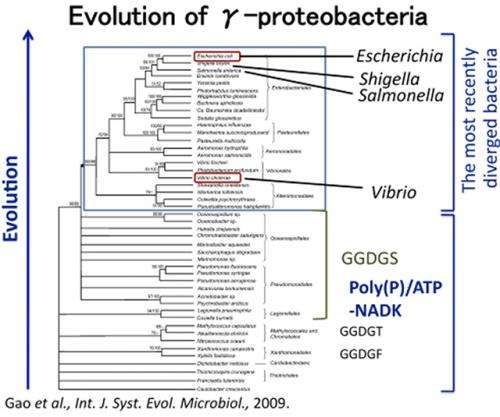
The group have assumed that NADKs evolved from poly(P)/ATP-NADKs into ATP-specific NADKs, and that recently diverged organisms obtained ATP-specific NADKs during their evolution, based on the following observations: (i) it has been deduced that bacteria evolved from Gram-positive bacteria or Archaea into Gram-negative ?-proteobacteria; (ii) poly(P)/ATP-NADKs are distributed throughout Gram-positive bacteria and Archaea, whereas ATP-specific NADKs are found in Gram-negative ?-proteobacteria (Escherichia coli and Salmonella enterica) and eukaryotes. However, it remains unclear how NADKs evolved from poly(P)/ATP-NADKs into ATP-specific NADKs.
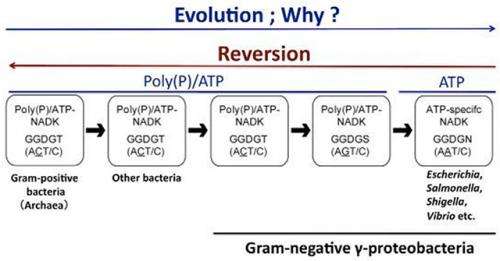
In this study, we succeeded in conferring the ability to utilize poly(P) on ?-proteobacterial ATP-specific NADKs, and hence in the effective "reversion" of these NADKs, through a single amino-acid substitution: substitution of Asn to Thr in the GGDGN motif that is conserved in the ?-proteobacterial ATP-specific NADKs. As a result of this substitution, the poly(P)-dependent NADK activity of E. coli ATP-specific NADK dramatically increased, from 2.8? to 43? (the relative activity to the 100? ATP-dependent activity). These data indicate that NADK has lost the ability to utilize poly(P), and that it acquired its specificity for ATP over the course of evolution as a result of the change from Thr to Asn in the GGDGT motif conserved among NADKs from almost all organisms.
Remarkably, almost all ?-proteobacterial NADKs carrying the GGDGN motif are further concentrated within the most recently diverged group within ?-proteobacteria, which includes E. coli and other pathogenic bacteria (Salmonella, Shigella, Yersinia, Vibrio, etc.) (Fig.2). Therefore, we propose that NADKs evolved from poly(P)/ATP-NADKs (carrying GGDGT) via Gram-negative ?-proteobacterial poly(P)/ATP-NADKs (carrying GGDGS) into Gram-negative ?-proteobacterial ATP-specific NADKs (carrying GGDGN) (Fig.2), as also depicted in Fig.3. It should be noted that human NADK carries the GGDGT motif, although this enzyme is ATP-specific, indicating that the mechanism underlying the acquisition of specificity for ATP (i.e., the structural determinants conferring specificity for ATP) differs between human ATP-specific NADK and ?-proteobacterial ATP-specific NADKs.
The group have established methods for large-scale production of NADP+, which is used in the diagnostic pharmaceutical industry, by utilizing poly(P)/ATP-NADK (Patent: 4088251 JAPAN). However, we have shown that the poly(P)/ATP-NADK created through the method described here exhibits higher poly(P)-dependent activity, and is therefore more suitable for poly(P)-dependent mass production of NADP+, than the poly(P)/ATP-NADK the group used originally.
Because the structural determinants conferring specificity to ATP differ between human ATP-specific NADK and ?-proteobacterial ATP-specific NADKs, it might be possible to develop new antibiotics that specifically target ?-proteobacterial ATP-specific NADKs, but not human ATP-specific NADK. This study also provides a significant clue regarding how NADKs recognize and utilize poly(P). This information opens the way to development of a novel system for production of valuable compounds, in which the ability to utilize poly(P) would be conferred on various ATP-specific enzymes, and the resultant poly(P)-dependent enzymes could then be utilized to produce various phosphoryl compounds using poly(P) instead of ATP. Finally, this study provides significant hints regarding why poly(P)/ATP-NADK has evolved into ATP-specific NADK, what the physiological significance of this evolution is, and what the relationship of this evolution with the properties (pathogenicity, etc.) of each bacterium is.
More information: Yusuke, N. et al. Conferring the ability to utilize inorganic polyphosphate on ATP-specific NAD kinase, Scientific Reports 3, 2632. DOI: 10.1038/srep02632 (2013)