New study identifies the signal that guides the migration and differentiation of enteric neuron precursors
Neural development involves the proliferation and migration of immature neurons, followed by their differentiation into the multiple cell types that make up the nervous system. These processes are poorly understood but are known to require the coordinated activity of dozens of transcription factors and signaling molecules. Hideki Enomoto, Toshihiro Uesaka and colleagues from the RIKEN Center for Developmental Biology have now identified a molecule that controls the migration and differentiation of a large population of neurons in the gut, known as the enteric nervous system (ENS).
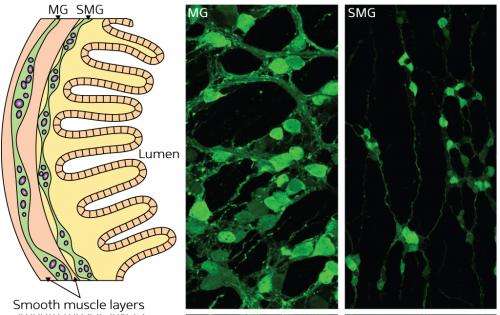
The ENS (Fig. 1) consists of two dense clusters of neurons: the myenteric ganglia (MG) and the submucosal ganglia (SMG). It is derived from the neural crest, a transient population of stem cells that arises along the top of the nervous system during early development. Although most of the peripheral nervous system forms relatively quickly, with cell migration followed immediately by differentiation, the ENS precursors that form the SMG are arrested in an immature state for a long period of time.
Glial cell line-derived neurotrophic factor (GDNF) is required for the proliferation and migration of premature ENS cells to form the MG. These cells are still responsive to GDNF at later stages of development but exactly why was unclear until now. Uesaka and his colleagues created a strain of genetically engineered mice in which the gene encoding the GDNF receptor can be inactivated at any time-point in development and made to express green fluorescent protein instead.
The researchers deactivated expression of the receptor at various time-points during ENS development and traced the labeled cells under the microscope. The study revealed that GDNF signaling is essential for both primary MG and secondary SMG formation. They found that ENS precursors retained in the MG exhibited minimal activation of the GDNF downstream pathway. In addition, ENS precursors lacking the GDNF receptor maintained their undifferentiated state.
The findings suggest that low levels of GDNF signaling are crucial for maintenance of the undifferentiated state in ENS precursors, and that GDNF has a long-term impact on the driving force of ENS-precursor migration and neuronal differentiation. This was confirmed in experiments involving the reactivation of the GDNF receptor, which led the cells to resume their migration and neuronal differentiation.
"Hirschsprung's disease is associated with the genes for GDNF and its receptor, and neural crest stem cell functions regulated by GDNF probably contribute to its pathogenesis," says Uesaka. "Understanding the mechanisms regulating precursor-cell maintenance and neuronal differentiation could facilitate the design of cell-replacement strategies."
More information: Uesaka, T., Nagashimada, M. & Enomoto, H. GDNF signaling levels control migration and neuronal differentiation of enteric ganglion precursors. The Journal of Neuroscience 33, 16372–16382 (2013). dx.doi.org/10.1523/JNEUROSCI.2079-13.2013