November 21, 2013 report
Multibeam femtosecond optical transfection for the ultimate brain interface
(Medical Xpress)—The robotic brain surgeon, featured in the 2013 movie "Enders Game" is no fictional brain-fixing machine. The open-source surgical platform, known as Raven II, has already starred in several brain procedures to date. It is not too hard now to imagine machines like this eventually installing brain controlled interfaces (BCIs). What is missing from this futuristic vision, is what happens at the business end, where the bots meet the brain. This unfolding drama, which began with crude electrode array stimulation, now parlays a combination of optical technologies that permits both transfection of neurons with interface machinery, and their subsequent control. A huge advance in automating the transfection part, and reducing the time it takes by orders of magnitude, has been reported today in Nature's Scientific Reports by a Scottish group from the University of Saint Andrews. Their new technology delivers DNA plasmids containing optical indicators and ion channels to individual neurons using arrays of femtosecond laser beams—and they can do this as fast as they can reach out and touch the neuron profiles on the screen in front of them.
Femtosecond laser pulses, by concentrating optical power into a short interval, combine exacting control with a minimum use of power. By implication, there is also a minimum of damage to surrounding tissue due to errant or otherwise prolonged irradiation. One difficulty with femtosecond lasers has been that an exotic system of free-space beam delivery optics is often called for. This is because the short pulses are significantly transformed by passage through standard fiber optics. As the authors now show, off-the-shelf instruments, like two-photon scanning or uncaging microscopes can be readily modified to perform fast, automated laser persuasion of cell membranes to allow DNA to slip inside.
In order to deliver various molecular constructs to single cells, protocols including manual injection, modified patch-clamping, lipofection, and electroporation have been developed. Unfortunately, these methods do not scale well if you want to hotwire a bunch of cells in a short time. Transfecting neighboring cells with different reporters or channels, or alternatively the same cell but sequentially with different elements, would be off the table with these methods. Trying to transfect neurons in the brain rather than large egg cells, and using naked DNA rather vector-based DNA, or RNA, involves additional considerations.
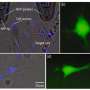
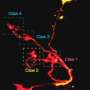
Using their custom-developed touchscreen, and image-guided femtobeam, the researchers were able to target up to 100 cells per minute. At a maximum recommended beam power of 77 milliWatts, they could also target a 4x4 array of points (on a 4um grid) to deliver 12-200 femtosecond pulses over 60 ms metapulse intervals. Depending on the specifics of the protocol, transfection yields from 50-100 percent could be obtained. These numbers were for dividing cells in which the nuclear membrane is transiently dispersed and therefore doesn;t present an additional barier to the DNA. For neurons, the researchers added a nuclear membrane-targeted peptide (Nupherin), that binds with the plasmid DNA and enhances transport. In further experiments with these neurons, they successfully activated the transfected channelrhodopsin protein using blue light, and recorded subsequently evoked spikes via patch clamp.
To really squeeze the technique into greater productivity, the researchers hope to implement spatial light modulators for precise and independent control of multiple beams. For an vivo or behaving scenario, the researchers point to fairly recent work where fiber based femotosecond transfection has been made to work in CHO-K1 cells at efficiencies of 74 percent. Using a compact, endoscope-like system with 6000 individual cores, this "nanosurgical instrument" was also used for simultaneous microfluidic delivery of drug to localized areas under direct imaging.
I asked lead author Maciej Antokowiak whether he thought there would be significant distortion in migrating to fiber-based delivery. He said that at 200fs, pulse stretching is much less of a concern than for the shorter 12-20 fs pulses. He also mentioned that in the high repetition regime (76MHz) femtosecond transfection appears to involve cumulative biochemical changes in the cell membrane.
Astounding reports of so-called glowing memories have also been trickling in this week along with the larger wake from the recent Society for Neuroscience meeting. This kind of selective optical interrogation of complete circuits in the brain will take mere connectomics into full-blown activity maps, and then, to control. As it has become apparent through omni-labelling techniques like Brainbow I and II, total label of the synaptic jungle is hardly better than no label. The ability to pick and choose multiple combinatorial activators or other modifiers, by finger or algorithm, as a prelude to thought itself, will be the quickest path to workable BCIs and our subsequent understanding of the brain.
More information: Fast targeted gene transfection and optogenetic modification of single neurons using femtosecond laser irradiation, Scientific Reports 3, Article number: 3281 DOI: 10.1038/srep03281
Abstract
A prevailing problem in neuroscience is the fast and targeted delivery of DNA into selected neurons. The development of an appropriate methodology would enable the transfection of multiple genes into the same cell or different genes into different neighboring cells as well as rapid cell selective functionalization of neurons. Here, we show that optimized femtosecond optical transfection fulfills these requirements. We also demonstrate successful optical transfection of channelrhodopsin-2 in single selected neurons. We extend the functionality of this technique for wider uptake by neuroscientists by using fast three-dimensional laser beam steering enabling an image-guided "point-and-transfect" user-friendly transfection of selected cells. A sub-second transfection timescale per cell makes this method more rapid by at least two orders of magnitude when compared to alternative single-cell transfection techniques. This novel technology provides the ability to carry out large-scale cell selective genetic studies on neuronal ensembles and perform rapid genetic programming of neural circuits.
© 2013 Medical Xpress