Trial continues after patient death: French heartmaker
French biomedical firm Carmat said Tuesday it would implant its experimental artificial heart into another three people, as planned, after the first recipient died.
In an email to AFP, the company said it was too soon to draw conclusions about the device's efficacy.
A 76-year-old recipient died over the weekend, 75 days after receiving the gadget, whose trials are being closely followed by French investors.
"It is premature to draw conclusions based on the outcomes from a single patient," said the the firm, whose stock was suspended at its own request on Tuesday.
"Carmat does not intend announcing the results of this trial until the implants and 30-day followup of all four intended four patients are complete."
The septuagenarian with terminal heart disease was one of four people, all with end-stage heart failure, selected for the experiment.
The trial would be considered a success if each of the four survive for at least a month.
Artificial hearts have been in use for many years as a temporary fix for patients with chronic heart problems.
Unlike these mostly synthetic pumps, the Carmat product aims to provide a longer-term solution—up to five years—to bridge the wait for a donor heart and enabling hospitalised patients to go back home and even resume work.
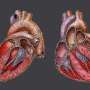
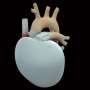
The device, a self-contained unit implanted in a patient's chest, is a mix of synthetic materials and animal tissue, and seeks to mimic the form and function of an actual human heart.
Nearly 100,000 people in Europe and the United States are in need of a heart transplant, according to Carmat.
© 2014 AFP