Likely culprit in spread of colon cancer identified
New research at Washington University School of Medicine in St. Louis and Vanderbilt University Medical Center in Nashville has implicated a poorly understood protein called PLAC8 in the spread of colon cancer.
While elevated PLAC8 levels were known to be associated with colon cancer, the researchers now have shown that the protein plays an active role in shifting normal cells lining the colon into a state that encourages metastasis.
The work appears April 1 in the Journal of Clinical Investigation.
"We knew levels of this protein are elevated in colon cancer," said co-author Lilianna Solnica-Krezel, PhD, professor and head of the Department of Developmental Biology at Washington University. "Now we've shown what PLAC8 could be doing—causing the cells to transition to a state that allows them to spread.
"This discovery developed from a collaboration between my group studying zebrafish and Robert Coffey's lab looking at human cells, both initially at Vanderbilt," she said. "Since 2010, my group has continued the zebrafish work at Washington University."
Senior author Robert Coffey, MD, the Ingram Professor of Cancer Research at Vanderbilt University, and his group have been developing new methods to grow colon cancer cells in three dimensions, rather than using typical procedures to grow cells in a flat dish.
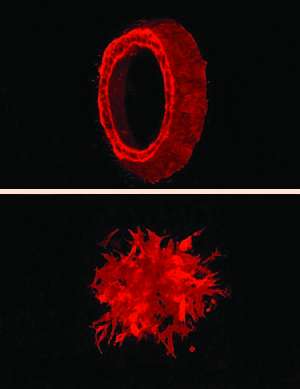
Coffey's group observed that colon cancer cells growing in three dimensions formed either smooth hollow balls or spiky clumps with protrusions extending into the surroundings. Compared to the smooth balls, the spiky clumps were shown to form rapidly spreading tumors in mice. When the researchers compared gene expression between the cells forming smooth balls and those forming spiky clumps, PLAC8 stood out. It was expressed at extremely high levels in the spiky clumps that formed aggressive tumors.
To gain a better understanding of PLAC8, Haiting Ma, PhD, a former graduate student in the Solnica-Krezel and Coffey labs, used a zebrafish model system to investigate the roles of this protein.
"We looked at this protein in zebrafish and saw that it was also expressed in the gut," said Solnica-Krezel. "In normal zebrafish, PLAC8 is present on the inner lining of the gut. We also noticed PLAC8 is heavily expressed in the early embryos of zebrafish."
Ma and his colleagues looked further into the developmental roles of PLAC8 and found that when there is too much of this protein, the zebrafish embryo developed abnormally, with slower cell movements resulting in an abnormal body shape and other developmental defects.
"We realized that these defects were very similar to abnormalities we see when the protein E-cadherin is mutated," Solnica-Krezel said. "E-cadherin is a cell adhesion molecule present on the cell surface, which allows cells to stick to one another. The amount of E-cadherin on the surface is very important for cell movement, with too much or too little being detrimental to mobility."
E-cadherin is also important in maintaining the sheet-like tissue structure called epithelium, which forms the inner lining of many organs, including the gut. Loss of E-cadherin can indicate a process known as epithelial-to-mesenchymal transition, where the cells detach, and the tissue loses its sheet-like nature, making it easier for the cells to migrate.
During early development, these transitions are normal, as cells must migrate to different parts of the developing organism and form new tissues and organs. But in cancer, this transition to more mobile cells can be the tipping point that causes them to break away from a tumor and invade neighboring tissues.
"Scientists know a lot about E-cadherin," Solnica-Krezel said. "But this is the first link between PLAC8 and E-cadherin. Nobody knew that PLAC8 could regulate it. Too much PLAC8 causes E-cadherin levels to go down, and low E-cadherin is associated with abnormal cell movement."
Moving full circle, first with human cells, then with zebrafish, the researchers returned to human tissues to investigate PLAC8 and associated proteins in colorectal tumors. They demonstrated that many markers of the epithelial-to-mesenchymal transition observed in zebrafish embryos with too much PLAC8 were also present at the edge of a human colon tumor.
Solnica-Krezel speculates PLAC8 could be an interesting target for future work in developing new cancer therapies.
"One could think about finding chemicals that might inhibit PLAC8's activity," she said. "But at present, this finding may have prognostic value. Those tumors expressing PLAC8 at high levels will be the most invasive."
More information: Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, Desai K, Gerdes MJ, Solnica-Krezel L, Coffey RJ. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. Journal of Clinical Investigation. April 1, 2014.