Mouse study points to potentially powerful tool for treating damaged hearts
A type of cell that builds mouse hearts can renew itself, Johns Hopkins researchers report. They say the discovery, which likely applies to such cells in humans as well, may pave the way to using them to repair hearts damaged by disease—or even grow new heart tissue for transplantation.
In a study to be published in an upcoming issue of the journal eLife, the scientists also found that during heart formation, these so-called cardiac progenitor cells (CPCs) multiply without becoming heart cells in a cellular environment known as the second pharyngeal arch. This insight into the biology of CPCs may contribute to better understanding of how to prevent and treat congenital heart defects, they say.
"Our finding that CPCs are self-renewing—that they can keep dividing to form new CPCs—means they might eventually be maintained in a dish and used to make specific types of heart cells," says Chulan Kwon, Ph.D., an assistant professor of cardiology and member of the Institute for Cell Engineering at the Johns Hopkins University School of Medicine. "Growing such cells in a dish would be an enormous step toward better treatment for heart disease."
Kwon's research group's first step was figuring out the role of two genes, Numb and Numbl, in CPCs, which others' studies had shown are needed for guiding stem and progenitor cells to their fully mature, specialized functions. Numb and Numbl are highly conserved, meaning that they're nearly identical in mice, humans and other animals, a sign that they're likely very important. To find out whether these genes are required for heart formation, the group disabled Numb and Numbl in early CPCs in developing mouse embryos. "The embryos failed to develop normal hearts and died at an early stage of development, showing us that Numb and Numbl are needed for CPCs to build the heart," Kwon says.
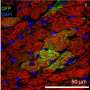
The researchers next set out to find where CPCs live in the developing embryo. Using embryonic stem cells from mouse embryos, they again disabled Numb and Numbl while also engineering the cells to produce a glowing red protein, which would give away the CPCs' location. But because the engineered stem cells alone wouldn't grow into a viable embryo, the team injected them into normal mouse blastocysts—a structure formed in the early stage of mammalian development that forms both the embryo and placenta. "The normal cells in these blastocysts compensated for those that lacked Numb and Numbl, allowing the resulting embryos to survive," Kwon says.
When the team checked the hearts of the embryos, they found the glowing red cells in the second pharyngeal arch, which is known for forming parts of the neck and face. Kwon says theirs is the first study to identify it as home to CPCs. His team took cells surrounding CPCs from this arch and grew them with CPCs in a dish. They found that the CPCs self-renewed without developing into specialized heart cells. This is an important step, he says, toward using CPCs to treat heart disease.
The next step, he says, is to coax the lab-grown CPCs to form new heart tissue that could be used to regenerate disease-damaged heart tissue. "Eventually, we might even be able to deliver cells to damaged hearts to repair heart disease," Kwon says.
More information: elifesciences.org/content/earl … 14/04/23/eLife.02164