Toward unraveling the Alzheimer's mystery
Getting to the bottom of Alzheimer's disease has been a rapidly evolving pursuit with many twists, turns and controversies. In the latest crook in the research road, scientists have found a new insight into the interaction between proteins associated with the disease. The report, which appears in the journal ACS Chemical Neuroscience, could have important implications for developing novel treatments.
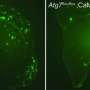
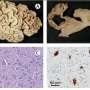
Witold K. Surewicz, Krzysztof Nieznanski and colleagues explain that for years, research has suggested a link between protein clumps, known as amyloid-beta plaques, in the brain and the development of Alzheimer's, a devastating condition expected to affect more than 10 million Americans by 2050. But how they inflict their characteristic damage to nerve cells and memory is not fully understood. Recent studies have found that a so-called prion protein binds strongly to small aggregates of amyloid-beta peptides. But the details of how this attachment might contribute to disease—and approaches to treat it—are still up for debate. To resolve at least part of this controversy, Surewicz's team decided to take a closer look.
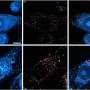
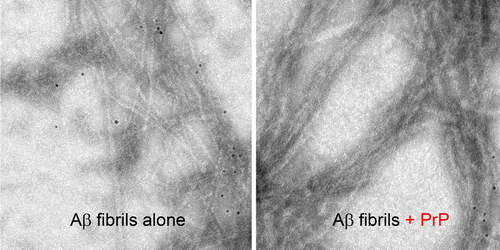
Contrary to previous studies, they found that the prion protein also attaches to large fibrillar clumps of amyloid-beta and do not break them down into smaller, more harmful pieces, as once thought. This finding bodes well for researchers investigating a novel approach to treating Alzheimer's—using prion-protein-based compounds to stop these smaller, toxic amyloid-beta pieces from forming, the authors conclude.
More information: "Interaction between Prion Protein and Aβ Amyloid Fibrils Revisited" ACS Chem. Neurosci., Article ASAP. DOI: 10.1021/cn500019c
Abstract
Recent studies indicate that the pathogenesis of Alzheimer disease may be related to the interaction between prion protein (PrP) and certain oligomeric species of Aβ peptide. However, the mechanism of this interaction remains unclear and controversial. Here we provide direct experimental evidence that, in addition to previously demonstrated binding to Aβ oligomers, PrP also interacts with mature Aβ fibrils. However, contrary to the recent claim that PrP causes fragmentation of Aβ fibrils into oligomeric species, no evidence for such a disassembly could be detected in the present study. In contrast, our data indicate that the addition of PrP to preformed Aβ fibrils results in a lateral association of individual fibrils into larger bundles. These findings have potentially important implications for understanding the mechanism by which PrP might impact Aβ toxicity as well as for the emerging efforts to use PrP-derived compounds as inhibitors of Aβ-induced neurodegeneration.