FDA approves first-of-a-kind sleep apnea implant

Sleep deprived Americans have a new option to address hard-to-treat nighttime breathing problems: a first-of-kind device that keeps airways open by zapping them with an electrical current.
The Food and Drug Administration approved the pacemaker-like device from Inspire Medical Systems for sleep apnea patients who have trouble with the current standard of care: machines that blow air through a bedtime mask.
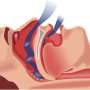
One of the main causes of sleep apnea is that the tongue and throat muscles relax too much during sleep, often blocking breathing and waking patients up. People who suffer from the condition lose crucial deep sleep time and are at higher risk for car accidents, heart attack and stroke.
Inspire's device treats the problem by stimulating a nerve that controls key airway muscles so that they stay in place, rather than flopping around and interfering with breathing.
Between 12 million and 18 million Americans have sleep apnea, according to the National Institutes of Health. It's particularly common in people who are overweight and in middle-aged men, but anyone can have it.
Today's first-choice treatment, called CPAP, uses special masks to gently blow air through the nose to keep airways open. But studies suggest roughly half of all patients that start CPAP do not consistently use it. They cite masks that fit poorly and leak, or say they feel claustrophobic, or rip them off while tossing and turning during the night.
The FDA approved the new technology from Minneapolis-based Inspire Medical Systems for patients with moderate to severe obstructive sleep apnea.
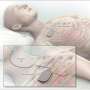
The company's system consists of a small generator, which is implanted in the upper chest region much like a pacemaker or a defibrillator. The generator is connected to an electrical stimulation lead in the throat that senses breathing patterns and delivers a current to keep airways open during sleep. Patients activate the system using a small hand-held remote before bed and then turn the system off when they wake up.
Company officials say the implant is less invasive and has a shorter recovery time than surgeries that are sometimes used to try and treat sleep apnea by removing part of the roof of the mouth or widening airways.
© 2014 The Associated Press. All rights reserved.