Cancer drug boosts levels of vascular-protective gene, KLF2
Case Western Reserve University researchers have discovered that an existing drug used to help cancer patients has the potential to protect thousands of others from the often-deadly impact of vascular clots.
In 2008, the Food and Drug Administration approved bortezomib (Velcade) to treat multiple myeloma, which is a type of bone cancer and mantle cell lymphoma—a particularly aggressive form of non-Hodgkin lymphoma. In addition to attacking cancer cells, the drug has been shown to help prevent clot development common to many forms of the disease.
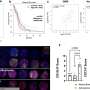
As hematologist Lalitha Nayak, MD, an assistant professor of medicine, reports in the June 12 edition of the journal Blood, the anti-thrombotic effects of bortezomib are determined by KLF2, part of a family of Kruppel-like factors—master regulators of vascular health.
"We thought that if we could figure out how bortezomib protects against thrombosis," Nayak explained, "we might be closer to understanding why our patients develop blood clots and what could be done to help them."
She is also a member of the Mukesh Jain, MD, Laboratory at Case Western Reserve University, so she was also well aware of the laboratory's work in Kruppel-like factors. KLF2 specifically is a protein in the Kruppel-like gene family of transcription factors that prevents clot formation in the body's major blood vessels. (Transcription factor is a protein that controls the flow of genetic information that provides instructions to our bodies on how to function.)
"Work from our laboratory during the past decade has established Kruppel-like factors as nodal regulators of vascular health," said Jain, cardiologist and professor of medicine. "It was a good educated guess by Dr. Nayak that bortezomib's positive effect on vascular function was linked to a member of this family."
Nayak concurs. "We hypothesized that bortezomib protects against thrombosis by increasing KLF levels," she said.
She took her curious, yet pleasantly surprising, observation regarding bortezomib's thromboprotective effect to the laboratory. In her investigations, Nayak first showed that bortezomib treatment rendered normal mice resistant to clot formation. Next, she demonstrated that among the 17 members of the KLF family, bortezomib specifically and potently induced KLF2 levels. Finally, she confirmed the importance of KLF2 by injecting bortezomib into mice missing the KLF2 gene. Although bortezomib treatment protected normal mice from thrombosis, this effect was absent in the KLF2-deficient mice. "This taught us how important KLF2 is for the ability of bortezomib to protect against thrombosis," Nayak said.
The results of this study have the potential to alter the management of thrombosis in patients who have a predisposition to clot formation and especially in situations where present modalities of therapy are inadequate. One example of such a condition is antiphospholipid antibody syndrome (APLS), a condition where patients have an increased risk for blood clots in both arteries and veins. The disease often affects young women, and there is no effective antithrombotic strategy for this group of patients. Moreover, currently used antithrombotic therapies are associated with an increased risk for bleeding. Although bortezomib protects against thrombosis, it comes with no concurrent increase in bleeding, making this drug a potential treatment alternative for APLS patients.
"Vascular clots are the No. 1 cause of death and disability worldwide," Nayak said. "Our studies show that increasing KLF2 levels provides a favorable thromboprotective effect."
At present, KLF2 levels can be pharmacologically altered with bortezomib. Importantly, the study showed that bortezomib can induce KLF2 levels and provide protection against thrombosis, even when used at much lower doses than those used to treat patients with myeloma.
"We are not trying to kill a cancer, so we started with smaller doses of the medication," Nayak said. "In our study, we were able to use one-third of the usual anti-tumor dose used in animal studies and found that this lower dose still resulted in a good antithrombotic effect."
Additionally, KLF2 itself could also serve as a biomarker, Jain said. "Examining KLF2 levels in blood cells might inform clinicians about a patient's risk for thrombotic events," Jain said. "This would help us identify patients who may benefit from upfront preventive therapy for thrombosis."