Diabetes susceptibility gene regulates health of cell's powerhouse
A team led by researchers from the Perelman School of Medicine at the University of Pennsylvania found that a susceptibility gene for type 1 diabetes regulates self-destruction of the cell's energy factory. They report their findings this week in Cell.
The pathway central to this gene could be targeted for prevention and control of type-1 diabetes and may extend to the treatment of other metabolic-associated diseases.
The team found that the gene, Clec16a, when in pancreas cells, is required for normal glucose-stimulated insulin release. What's more, people with a variation in the gene sequence near Clec16a have reduced expression of the protein in their islet cells and therefore reduced insulin secretion.
First author Scott A. Soleimanpour, MD, a postdoctoral fellow in the lab of co-senior author Doris Stoffers, MD, PhD, professor of Medicine, worked out this role of Clec16a in pancreatic beta cells. Soleimanpour is now an assistant professor at the University of Michigan Medical School. Stoffers is also a member of the Institute for Diabetes, Obesity, and Metabolism at Penn.
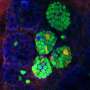
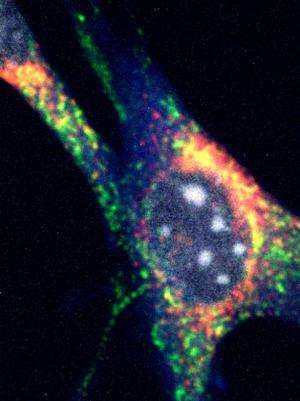
The self-destruction process of the cell's energy factories (mitochondria) is called mitophagy. This literally means the self-eating of mitochondria, the sites for producing the energy molecule ATP. Beta cells within the pancreas are enriched in mitochondria because of their insulin-secreting function, an energy-intensive process.
Mitophagy involves the breaking down and recycling of less well-functioning, old mitochondria to build fresh ones. Clec16a controls beta-cell function in this disposal pathway and is thought to prevent diabetes-related mitophagy.
Little had been known about the function of the Clec16a protein in mammals or of its role in the initiation of disease. The team found that Clec16a interacts with an enzyme called Nrdp1, which works through another protein called Parkin. Normally, Parkin regulates mitophagy by initially tagging unhealthy mitochondria for disposal.
Mice with a pancreas-specific deletion of Clec16a have abnormal mitochondria that produce less ATP, which is required for normal beta cell function, and ultimately insulin secretion. Specifically, they determined that the loss of Clec16a leads to an increase in Parkin, a master regulator of mitophagy. What's more, the team found that the final disposal of unhealthy mitochondria was also defective.
"The ultimate result of the deletion of Clec16a is an accumulation of unhealthy mitochondria, leading to less insulin being secreted by the beta cells," says Stoffers.
Coauthors from the Lund University and Skåne University Hospital in Sweden provided a panel of human islet cells that allowed Soleimanpour to determine whether a small diabetes-risk variation in the DNA sequence near the Clec16a gene directly affected the gene's normal expression and function. Individuals with this short sequence variant had reduced Clec16a expression in islet cells, as well as slightly elevated blood sugar. In addition, tapping into a large, previously published genetic database allowed the investigators to further correlate the same disease-associated sequence variant with reduced beta-cell function. From this the team concluded that, in a normal state, Clec16a controls beta cell function and prevents diabetes by controlling mitophagy.
"In 2007, our genomics team found the first gene in a genome-wide search to play a major role in type 1 diabetes, but we did not know its function," said co-senior author Hakon Hakonarson, MD, PhD, director of the Center for Applied Genomics at The Children's Hospital of Philadelphia. "Now we understand how this gene plays a critical role in regulating insulin metabolism."
The novel Clec16a pathway could be targeted for prevention and control of diabetes and may extend to the pathogenesis of other Clec16a and Parkin-associated diseases, conclude the researchers.