Team finds switch that causes mature liver cells to revert back to stem cell-like state
Harvard Stem Cell Institute scientists at Boston Children's Hospital have new evidence in mice that it may be possible to repair a chronically diseased liver by forcing mature liver cells to revert back to a stem cell-like state.
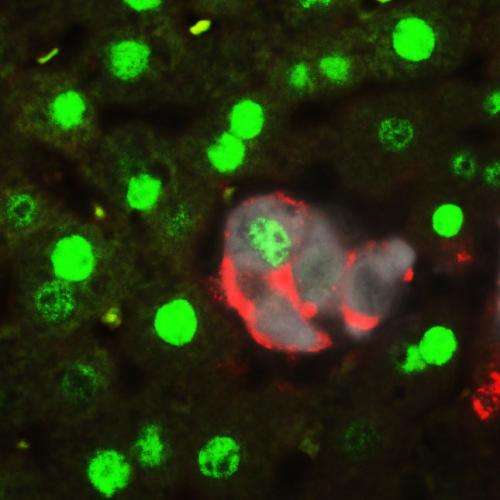
The researchers, led by Fernando Camargo, PhD, happened upon this discovery while investigating whether a biochemical cascade called Hippo, which controls how big the liver grows, also affects cell fate. The unexpected answer, published in the journal Cell, is that switching off the Hippo-signaling pathway in mature liver cells generates very high rates of dedifferentiation. This means the cells turn back the clock to become stem-cell like again, thus allowing them to give rise to functional progenitor cells that can regenerate a diseased liver.
The liver has been a model of regeneration for decades, and it's well known that mature liver cells can duplicate in response to injury. Even if three-quarters of a liver is surgically removed, duplication alone could return the organ to its normal functioning mass. This new research indicates that there is a second mode of regeneration that may be repairing less radical, but more constant liver damage, and chips away at a long-held theory that there's a pool of stem cells in the liver waiting to be activated.
"I think this study highlights the tremendous plasticity of mature liver cells," said Camargo, who is an associate professor in the Harvard Department of Stem Cell and Regenerative Biology, and based in the Stem Cell Program at Boston Children's Hospital. "It's not that you have a very small population of cells that can be recruited to an injury; almost 80 percent of hepatocytes [liver cells] can undergo this cell fate change."
Much of the work dissecting the biology of these changes and establishing that the dedifferentated cells are functional progenitors was carried out by the Cell paper's first co-authors Dean Yimlamai, MD, PhD, and Constantina Christodoulou, PhD, of Boston Children's Hospital.
The next step, Camargo said, would be to figure out how Hippo's activity changes in cells affected by chronic liver injury or diseases such as hepatitis. In the long term, this work could lead to drugs that manipulate the Hippo activity of mature liver cells inside of patients to spur dedifferentiation and hasten healing.
It might also be possible to control Hippo signaling to grow countless liver progenitor cells in a laboratory dish for transplant, which Camargo's team pursued in the Cell paper using mice born with a genetic liver disease. They cultured healthy liver progenitor cells and transplanted them into the diseased mice. Over a period of three or four months, the transplanted liver cells engrafted and the animals saw improvement of their condition.
"People have been trying to use liver cell transplants for metabolic diseases since the early 90s, but because of the source of cells—discarded livers—they were unsuccessful," Camargo said. "With this unlimited source of cells from a patient, we think that perhaps it's time to think again about doing hepatocyte or progenitor cell transplants in the context of liver genetic disorders."
The observation that mature liver cells dedifferentiate comes after a number of related studies published in the past year from Harvard researchers showing that mature cells in several different internal organs, including the kidneys, adrenal glands, and lungs, are more plastic than we once assumed.
"I think that maybe it is something that people have overlooked because the field has been so stem cell centric," said Camargo, also a Harvard Stem Cell Institute Principal Faculty member. "But I think the bottom line is that the cells that we have in our body are plastic, and understanding pathways that underlie that plasticity could be another way of potentially manipulating regeneration or expanding some kind of cell type for regenerative medicine."
More information: Yimlamai et al., Hippo pathway activity influences liver cell fate, Cell (June 5, 2014), dx.doi.org/10.1016/j.cell.2014.03.060