Computation and collaboration lead to significant advance in malaria
Researchers led by Baylor College of Medicine have developed a new computational method to study the function of disease-causing genes, starting with an important new discovery about a gene associated with malaria – one of the biggest global health burdens.
The work published today in the current issue of the journal Cell includes collaborators comprised of computational and evolutionary biologists and leading malaria experts from Baylor, Columbia University Medical Center, Princeton University, Pennsylvania State University and the National Institute of Allergy and Infectious Diseases (NIAID).
"Today, rapidly falling costs means that high throughput sequencing projects are revealing the entire gene sequences of ever more species, but the biological functions of most of these genes remain unknown," said Dr. Olivier Lichtarge, professor of molecular and human genetics and director of the Computational and Integrative Biomedical Research Center at Baylor and senior author of the report. "To address this problem, our lab has developed new methods to predict gene and protein functions."
Dr. Andreas Martin Lisewski, an instructor in Lichtarge's lab at Baylor, served as the leading author on the report.
The researchers came up with a computational method that allows biological information to literally flow from gene to gene across a massive network across many genomes, known as the "supergenomic" network.
"The network connects millions of genes from hundreds of species based on their interactions within the organism or based on their ancestral relations between different species," said Lisewski. "Normally, computing the flow of functional information would be costly and slow, but we developed a compression method that reduces this gigantic network into one that is much smaller and now computationally tractable. The surprise is that these biological networks are compressible much like digital data in today's computers."
To test their method, the researchers looked at functional predictions of a protozoan parasite known to cause the most severe form of malaria in humans – Plasmodium falciparum. While it has been more than 10 years since the genome of this parasite was fully sequenced, still too little is known about the function for most of its genes.
Every year, malaria affects more than 200 million people and contributes to nearly 1 million deaths worldwide.
"To better understand this disease, we need to identify more functions of the parasite's genes. This understanding may eventually help us to stem the rise of drug-resistant malaria, such as the emerging resistance to artemisinins," said Lisewski.
Artemisinins are a family of drugs that currently form the frontline treatment against Plasmodium falciparum malaria. Artemisinin was originally isolated as an extract from a traditional Chinese herbal remedy, and while it is still highly effective against malaria in patients, the mechanism of action has been unclear. A loss of artemisinin's antimalarial effectiveness due to genetic resistance would have devastating global health consequences.
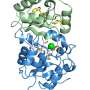
The researchers honed in on the parasite protein EXP1 that was known to be essential to the malaria parasite but for which there were no details on its function.
Using the network, they showed that this protein enables the parasite to detoxify the main metabolic byproducts it creates in red blood cells. They also demonstrated that it has a direct role in drug action and susceptibility to artesunate, an important member of the artemisinin drug family.
"Through this multi-year collaborative effort, we now have an improved understanding of the protective molecular mechanisms of the malaria parasite and its drug susceptibility to artesunate. As we are witnessing a rise of resistance to artemisinins, these results may help finding new pathways to successor drugs," said Lichtarge.