August 29, 2014 feature
Medicinal GPS: DNA nanotechnology-based approach allows injected drugs to find tumor sites
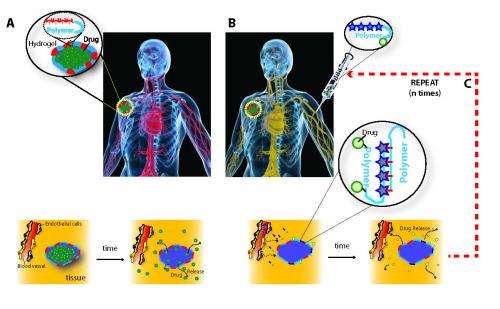
(Medical Xpress)—Current therapies for cancer, wound healing, inflammation, and many other diseases – as well as protocols for drug reloading of vascular grafts and stents – often rely on so-called drug delivery depots, which are infused with medication and surgically implanted proximate to the site being treated. However, drug delivery depots in clinical use today are single-use, with no ability to be refilled once exhausted. Moreover, once all medication has been released they sometimes require removal by way of additional invasive surgery, thereby exposing the patient to additional risk. A long-sought solution to these drawbacks is localized drug delivery that would allow for minimally invasive refilling of drug depots for repeat drug dosing over the course of weeks or months. Refilling local drug delivery would also obviate the need for surgical removal. Recently, scientists at Wyss Institute for Biologically Inspired Engineering, Harvard University devised a DNA nanotechnology-based approach for blood-based drug refilling of hydrogel intratumor drug depots using nucleic acid sequence complementarity. More specifically, oligodeoxynucleotide-conjugated alginate drug payloads were used for refilling drug-delivering hydrogels containing the complementary sequence and exploited for tumor treatment. (An oligodeoxynucleotide, or ODN, is a short sequence of nucleotides – RNA or DNA – that contain deoxyribose; alginate is an anionic polysaccharide distributed widely in the cell walls of brown algae.) The researchers conclude that their proof-of-concept study demonstrates the potential application of refilling other drug-delivery devices in the treatment of a wide range of diseases.
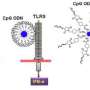
Dr. Yevgeny Brudno discussed the paper that he, Prof. David J. Mooney and their co-authors published in Proceedings of the National Academy of Sciences with Medical Xpress. In developing, the scientists faced three main challenges: designing a system that exploits nucleic acid complementarity to refill drug-delivering depots through the blood; modifying an injectable delivery device to bind refills infused into the blood; and using alginate for both local controlled drug release and blood-based targeting. "The main challenges to exploiting DNA nanotechnology for drug refilling was to formulate alginate and DNA conjugates that both bound specifically to and were stable in vivo for use with drug delivery devices," Brudno tells Medical Xpress. "Our drug-alginate-DNA formulations had to have good pharmacokinetics, in vivo stability and the ability to specifically recognize and bind to the device resident at a disease site."
Pharmacokinetics – a drug's sequential absorption, bioavailability, distribution, metabolism, and excretion – involves receptor binding, post-receptor effects and chemical interactions, and therefore determines the onset, duration and intensity of a drug's effect. "The formulation that we eventually settled on solved these challenges through the use of chemically-stabilized DNA conjugated to alginate strands with good pharmacokinetics."
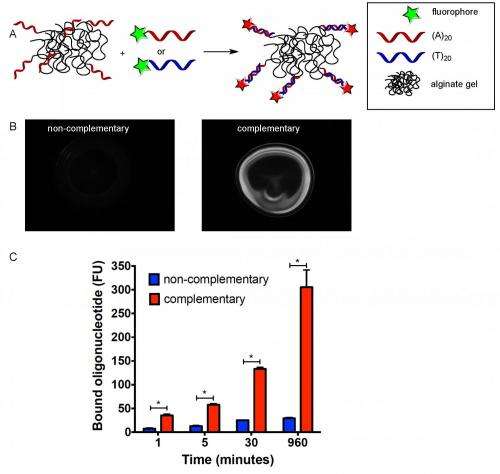
The fact that local drug-delivering devices could not be refilled has been a major limitation in the field, and served to motivate the current investigation. "The major insight of this work was that these devices could serve as a 'homing beacon' for blood-based nanoparticles," he notes. "One major innovation of this work was to use nucleic acid recognition, allowing for specific binding between the drug-carrying nanoparticle refills and implanted devices."
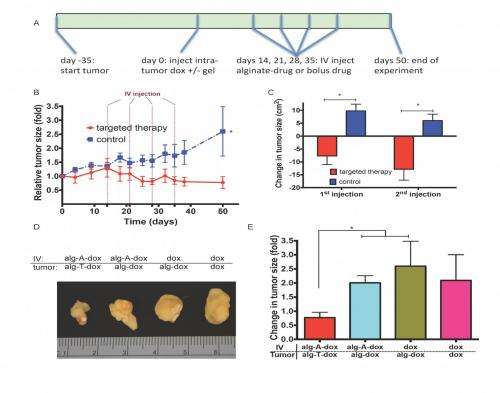
The paper's key results describe the new approach's ability to inhibit tumor growth to a greater extent than strategies that rely on enhanced permeability and retention alone; drug payloads infused into a patient's blood extravasating – that is, being forced out – into target tissues and becoming bound by the device; and selective retention of refilling payloads reducing off-target toxicity in EPR (Enhanced Permeability and Retention) effect-based therapies. (The EPR effect is the property by which certain sizes of liposomes, nanoparticles, macromolecular drugs and other molecules accumulate in tumor tissue much more than they do in normal tissues.) "The two most common drug delivery technologies currently under development for cancer," Brudno explains, "are a local drug-delivering device, implanted in or close to the target site, which releases the drug directly at the site; and nanoparticles that carry drugs and accumulate in the tumor passively through the EPR effect. We compared our system to both of these technologies – as well as the clinical standard of infusing drugs into the blood – and found that our system performed significantly better than all of these existing technologies."
That said, while drug-carrying nanoparticles are known to specifically accumulate at tumor sites through the EPR effect, this accumulation is transient. "What we've shown is that we can significantly improve the amount of time that nanoparticles spend at the tumor site," Brudno adds. "This increased resident time allows the nanoparticle to release more of its drug cargo, and moreover the drug cargo is released over a greater time, translating to improved cancer therapy."
The paper notes that imaging of individual organs revealed accumulation mainly in the liver (as is the case with a range of nanoparticles) and kidneys. Since accumulation in the kidneys is likely due to the degradation of the oxidized material over time, which releases smaller alginate particles capable of being filtered by the kidneys, Brudno addressed the question of their approach being suitable for patients with structural or functional nephropathy, autoimmune diseases or compromised immune systems. "Drug-delivery systems based on local drug delivery or nanoparticle delivery are designed to overcome many of the toxicities associated with current cancer therapy," he tells Medical Xpress. "Our technology, which marries the two technologies of local and nanoparticle delivery, has proven significantly better than either alone at treating cancer in an animal model – and its reduced toxicity will benefit patients with all sorts of issues, including nephropathy and compromised immune systems."
Moving forward, the researchers are applying the principles of refillable drug-delivery devices to a variety of disease models. "We are actively pursuing refillable drug-delivery device in the context of wound healing," Brudno illustrates, "and are also investigating refilling the surfaces of drug-eluting vascular stents, vascular grafts and catheters." Because the refilling system is based on nucleic acid recognition, the scientists are focusing on the prospect of delivering multiple drugs specifically to multiple different sites on a patient – something not possible with current technology.
In addition to their DNA nanotechnology-based approach being a new drug-delivery paradigm for cancer therapy and other diseases, the scientists say their approach could also be used to specifically monitor activity in the body to prevent disease or perhaps to diagnose disease earlier than currently possible. "Refillable drug delivery devices," Brudno concludes, "could be converted to monitoring devices that are regularly refilled with molecules necessary for their activity."
More information: Refilling drug delivery depots through the blood, Proceedings of the National Academy of Sciences Published online before print August 19 2014, doi:10.1073/pnas.1413027111
© 2014 Medical Xpress