Skin disease drug finally wins approval
A synthetic hormone developed years ago at the UA can now be marketed in Europe, and U.S. approval will be sought next.
A synthetic hormone developed more than two decades ago by faculty at the University of Arizona Cancer Center and the UA's Colleges of Medicine and Science, shown to offer relief for a rare skin disease, has been approved for patients in Europe.
Clinuvel Pharmaceuticals Ltd. of Melbourne, Australia, received permission in October from the European Medicines Agency to market the drug, whose trade name is Scenesse. The company says it plans to seek approval for Scenesse from the U.S. Food and Drug Administration and also for patients in the Asia-Pacific region.
The molecule was developed, patented and initially tested at the UA under the name afamelanotide by Regents' Professor Emeritus of Chemistry and Biochemistry Victor Hruby; Robert Dorr, leader of the UACC's therapeutic development scientific program and professor of medical pharmacology in the UA's College of Medicine-Tucson; and the late Mac Hadley, a cell biology and anatomy professor. The UA licensed the molecule to a predecessor company, Melanotan Inc., in 1995.
Hruby said the process "involved the very close collaboration of chemists, biologists and medical doctors who brought similar excitement and creativity to the problem but from different perspectives." Dorr said this is the first approval of a pure melanotropin, which is a hormone that causes dispersion of melanin and results in darkening of the skin.
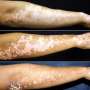
Scenesse has been demonstrated to reduce and prevent painful reactions to sunlight experienced by people with erythropoietic protoporphyria, or EPP, after trials in Europe, Australia and the U.S. EPP is a rare genetic disease found mainly in those with fair skin, characterized by intolerance to light and resulting in pain, swelling, burning and scarring of sun-exposed areas of skin. Those with this condition often must remain indoors during daylight hours.
As many as 10,000 suffer from EPP worldwide, with approximately 45 percent of them in Europe. EPP is considered the most common form of skin disorder in children.
Afamelanotide, formerly known as Melanotan I, is a superpotent form of the naturally occurring alpha-Melanocyte Stimulating Hormone, which stimulates melanin production. Melanin is known for its photoprotective effect on the skin. Initial proof-of-principle clinical trials were conducted at the UA Cancer Center under the Chemoprevention of Skin Cancer program project grant, led by Dr. David S. Alberts. Initial study results were published in the Journal of the American Medical Association in 1991.
Afamelanotide originally was developed by UA scientists as a defense against skin cancer, Dorr said. Stimulating a person's natural photoprotective skin pigmentation, melanin, would create tanned skin prior to sun exposure, protecting the person from harmful UV rays.
Clinuvel also is testing Scenesse as a treatment for several other skin conditions.