SU2C-supported research discovers why patients respond to a life-saving melanoma drug
Work supported by the Stand Up To Cancer (SU2C) - Cancer Research Institute (CRI) - Immunology Translational Research Dream Team, launched in 2012 to focus on how the patient's own immune system can be harnessed to treat some cancers have pioneered an approach to predict why advanced melanoma patients respond to a new life-saving melanoma drug. This new drug, pembrolizumab (Keytruda), was recently approved by the FDA. These findings are reported in Nature online November 26, 2014, ahead of print in the journal.
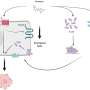
Over a two-year study, researchers including Dr. Antoni Ribas of UCLA Jonsson Cancer Center and co-leader of the CRI-SU2C Immunology Dream studied 46 patients with advanced melanoma treated with pembrolizumab. These patients had tumor biopsies before and during treatment. The researchers analyzed those biopsies and classified them according to whether the patient responded or not to pembrolizumab. The study then used the biopsy findings to predict the likelihood whether patients would likely to respond to this treatment
According to the press release issued by UCLA Jonsson Comprehensive Cancer Center, a protein known as PD-1 puts the immune system's brakes on, preventing T cells from attacking cancer cells. Pembrolizumab removes the brake lines, freeing up the immune system to kill cancer cells. Keytruda was the first PD-1 immunotherapy drug approved by the FDA in September 2014.
"The work of Dr. Ribas, his colleagues and the SU2C-CRI Immunology Dream Team is so important, bringing tangible benefits to patients struggling with advanced melanoma and providing the means to assess why some patients are responsive to this breakthrough drug," said Phillip A. Sharp, Ph.D., chairperson SU2C Scientific Advisory Committee, and MIT Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge. "Not only has this research validated a pathway to determine which patients may be responsive to pembrolizumab, we anticipate it will significantly inform development of better drug combinations that are more effective, less costly and with fewer side effects for even more patients with melanoma and other cancers."
More information: PD-1 blockade induces responses by inhibiting adaptive immune resistance, Nature 515, 568–571 (27 November 2014) DOI: 10.1038/nature13954