'Digitizing' crosstalk among heart cells may help locate epicenters of heart rhythms
A team of scientists led by Johns Hopkins cardiologist and biomedical engineer Hiroshi Ashikaga, M.D., Ph.D., has developed a mathematical model to measure and digitally map the beat-sustaining electrical flow between heart cells.
The work, the scientists say, could form a blueprint for vastly more precise imaging tests that capture cell-to-cell communication and pinpoint the tiny clusters of cells at the epicenter of complex, life-threatening arrhythmias. Such imaging approaches, they add, would enable precision-targeted, minimally invasive treatments that eliminate rhythm-disrupting hotspots in the heart's electrical system.
The approach, described online March 4 in the Journal of the Royal Society Interface, is inspired by so-called information theory and built on the premise that cell-to-cell interaction follows a classic model of communication consisting of source, transmitter and receiver. Translating those cellular "conversations" into digital form—a series of zeroes and ones that can be easily read and imaged by a computer—can help spot breakdowns in communication that form the epicenters of dangerous rhythm disturbances.
"Successful arrhythmia treatment depends on correctly identifying the epicenter of the malfunction," Ashikaga says. "We cannot begin to develop such precision-targeted therapies without understanding the exact nature of the malfunction and its precise location. This new model is a first step toward doing so."
At the heart of the new model is the idea that heart muscle cells act as analog-to-digital converters, taking up information from their surroundings, converting or interpreting the information, and transmitting the message to neighboring cells. Ashikaga and colleagues say that capturing and quantifying information transmitted from cell to cell can help "catch" aberrant signals—or communication breakdowns—as they trigger electrical firestorms that cause the heart to beat abnormally and compromise its ability to pump blood.
The location of such communication glitches has been notoriously challenging to pinpoint with standard electrocardiograms, or EKGs, which provide limited information and are most helpful in diagnosing the type of arrhythmia rather than the exact cellular origin of the rhythm disturbance.
In their new model, the researchers mapped cellular information flow by creating computer representations of normal and abnormal heartbeats, ranging from simpler benign arrhythmias with well-defined epicenters to dangerous rhythms that arise in multiple hotspots. The scientists then "digitized" the electric flow by converting the electrical signals transmitted by cells into bits—the zeroes and ones that are the basic units of information in computing and digital communications.
Next, they measured how much information was generated, transmitted and received during normal and abnormal heart rhythms and plotted the information onto a 2-D map to create an image of the arrhythmia.
The different types of arrhythmias generated markedly distinct spatial profiles. By contrast, regular EKG tracings of the same rhythm disturbances looked similar with a lot of overlapping features, an observation suggesting that quantifying and digitizing information flow inside the heart would far more reliably distinguish one form of arrhythmia from another.
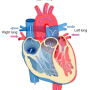
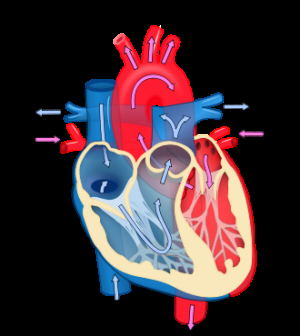
The human heart is a muscle made up of 5 billion cells loosely linked together by so-called gap junctions that carry electrical signals from one cell to the next, a perfectly synchronized process that culminates in a heartbeat. At times, however, the propagation of the electrical signal fails due to an anatomic roadblock in the heart muscle—such as scarring from a heart attack—or because of chemical imbalances that lead to electric malfunction. Often, the misfiring cells can self-correct quickly, restoring the electric flow. But now and then, the aberrant signal can propagate into a cluster of neighboring cells and spark heart rhythm disturbances, some of them serious and some life-threatening. Two of those arrhythmias—atrial fibrillation and ventricular fibrillation—pose the gravest risk to health. Atrial fibrillation, which affects 6 million Americans, can lead to blood clot formation and stroke. It occurs when the heart's upper chambers start beating chaotically. Ventricular fibrillation, an often-lethal rhythm disturbance responsible for 150,000 cardiac arrests each year, occurs when the normal pumping activity of the heart's lower chambers degenerates into weak, quivering beats.
Current therapies for dangerous rhythms, including medication, catheter ablation or implanted defibrillators that shock the heart back into normal rhythm, are not always effective or have serious downsides. But pinpointing the origin of dangerous arrhythmias could lead to new therapies and improve the precision of surgical ablation, a minimally invasive procedure that uses heat energy to burn the hotspots that trigger aberrant rhythms. Ablation works well for simple rhythm disorders with a well-defined hotspot, but is far less effective for complex arrhythmias originating from multiple hotspots that cannot be precisely located with standard imaging techniques.