Drug approved to treat osteoporosis shows promise in pre-clinical diabetes research
American scientists have discovered that a drug commonly used to treat osteoporosis in humans also stimulates the production of cells that control insulin balance in diabetic mice. While other compounds have been shown to have this effect, the drug (Denosumab) is already FDA approved and could more quickly move to clinical trials as a diabetes treatment. The research is published June 18 in Cell Metabolism.
Diabetes is a major health issue worldwide that arises due to a deficiency of insulin-producing beta cells in the pancreas. In type 1 diabetes, beta cells die from a misguided attack by the body's immune system; in type 2 diabetes, the body becomes resistant to insulin and beta cells try to compensate by producing more of it, which can wear them out. Therefore, a primary goal to combat diabetes is to find ways to increase functioning beta cells; however, adult beta cells are very resistant to divide and grow.
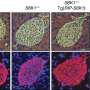
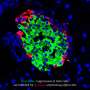
In addressing this hurdle, investigators have discovered a link between a well-known bone-related pathway and the proliferation of pancreatic beta cells. "Our study identifies a molecular brake that inhibits both mouse and human beta cell replication," says senior author Rupangi Vasavada of the Diabetes, Obesity and Metabolism Institute at the Icahn School of Medicine at Mount Sinai in New York City. "It shows that two proteins, including an FDA-approved osteoporosis drug, can override and release this brake to induce proliferation of rodent and human beta cells."
Vasavada and her colleagues, including study first author Nagesha Kondegowda, made their discoveries by studying the effects of lactogenic hormones, which are produced by the pituitary gland, stimulate lactation in females, and are known to enhance pancreatic beta cell survival and growth. When they looked for proteins that are regulated by lactogens in beta cells, the researchers identified a bone-related protein called Osteoprotegerin (OPG). Interestingly, a search of the medical literature revealed that OPG is expressed at high levels under various conditions—such as pregnancy and obesity—that promote beta cell expansion. These findings suggest that OPG may be directly involved in beta cell growth.
OPG binds to a protein and receptor pair that affects bone turnover, lactation, and a variety of other processes. Vasavada and her team found that the pair also inhibits beta cell replication and that OPG and Denosumab, which is an antibody, both counteract this effect to stimulate beta cell proliferation. "The findings suggest that there is potential for repurposing this osteoporosis drug for the treatment of diabetes," she says.
Vasavada will next explore how Denosumab and OPG modulate beta cell growth and function. She is also interested in doing clinical trials in patients with diabetes who are also being treated for osteoporosis with Denosumab or other drugs. A 2013 study of postmenopausal women taking Denosumab found no effect on glucose metabolism for those on the drug, but more tests are needed as the participants were not diabetics.
More information: Cell Metabolism, Kondegowda et al.: "Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-κB Ligand (RANKL) Pathway" dx.doi.org/10.1016/j.cmet.2015.05.021