Researcher helps identify host factor essential for malaria parasite to infect human red blood cells

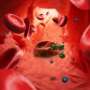
Red blood cells are a prime target for infection by the malaria parasite, but the absence of a nucleus containing DNA in red blood cells hinders genetic research to understand how these cells act as host cells.
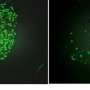
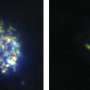
Seeking to help overcome that obstacle, researchers at Harvard T.H. Chan School of Public Health, The Broad Institute of Harvard and Massachusetts Institute of Technology, and the University of South Florida developed an innovative genetic screening technology to identify critical host factors required for the invasion and growth of the most deadly malaria parasite, Plasmodium falciparum, in red blood cells. The technology uses red blood cells derived from gene-silenced hematopoietic stem cells.
In a study published last month in Science, the research team discovered that a rare blood group known as CD55 is essential for the invasion of human red blood cells by P. falciparum.
"Now we have a technology to make mutated red blood cells," said the paper's second author Rays Jiang, PhD, assistant professor in the Department of Global Health, USF College of Public Health, and the Florida Center of Excellence for Drug Discovery and Innovation at USF.
"This will help us to understand how stem cells develop into mature blood cells, how malaria invades blood cells and, ultimately, to stop malaria infection."
The recent finding of CD55's critical role in infection – and the lack of toxicity associated with its absence in some individuals—suggests that this protein could be an attractive target for developing host-based malaria therapies less susceptible to drug resistance.
"Instead of killing the bug, maybe we target the host to make the parasite harmless," said Dr. Jiang. "We may be able to treat the disease by using a CD55 decoy protein to block the malaria parasite's critical entry point into the red blood cell."
Once P. falciparum has attacked red blood cells, it rapidly creates more parasites that spread in waves throughout the human host's bloodstream leading to the symptoms of severe malaria – fever, chills, loss of blood, brain damage and coma.
A mosquito-borne infectious disease, malaria affecting 10 percent of the world's population, killing nearly one million people a year in developing countries and crippling their economies. The form of the malaria caused by P. falciparum is a leading cause of death among children worldwide.
Dr. Jiang worked as computational biologist at the Broad Institute of Harvard, MIT and Harvard University before joining the College of Public Health's Global Infectious Diseases Research Program in 2014. Her interdisciplinary research team at USF includes expertise in infectious diseases, engineering and drug discovery as well as computational biology.
Dr. Jiang's scholarly work has contributed to understanding the global diversity of malaria parasites and the interplay of genetics and environmental factors in controlling the spread and severity of malaria. She develops and applies methods to analyze and interpret the enormous amounts of data generated by high-throughput biology, which automates the precise repetition of experiments on a large scale so researchers can rapidly study how cells function and interact with each other and how disease-causing microbes exploit them.
Working with principal investigator John Adams, PhD, a distinguished USF professor of global health, Dr. Jiang is a co-investigator for a five-year RO1 grant from the NIH's National Institute of Allergy and Infectious Diseases. The study uses chemogenomic profiling to identify P. falciparum's responses to screened drug candidates and to help rationally design more effective antimalarial treatments less likely to lead to drug resistance.
More information: "A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion." Science, 8 May 2015: Vol. 348, No.6235 pp. 711-714, DOI: 10.1126/science.aaa3526.