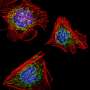
Automatable method lets researchers follow the paths of each mitochondrion in a cell
Mitochondria, the tiny oxygen reactors that power our cells, can be the keys to health or disease. But while the recognition of their importance has soared in recent years, methods for studying them haven't always kept pace. Now, scientists from the Perelman School of Medicine at the University of Pennsylvania have devised a powerful new technique that enables the tracking of every mitochondrion as it moves within a cell.
"We established a new framework for interrogating a key feature of metabolic performance, which is how mitochondria move about within a cell," said senior author David M. Eckmann, MD, PhD, the Horatio C. Wood Professor of Anesthesiology and Critical Care and professor of Bioengineering at Penn. "We can now use this technique when provoking aberrations of cellular health in a variety of ways to study disease and disease treatment."
"Already we've been able to use the new method to discover things about mitochondrial motility and how it can change on the whole-cell level," said the study's first author Judith Kandel, a graduate student in Eckmann's laboratory. Their findings are published in the July issue of Biotechnology and Bioengineering. Also among the authors is Philip Chou, a Penn undergraduate student who works in Eckmann's laboratory.
Researchers are increasingly aware that the disruption of normal mitochondrial motility may be not only a sign, but also a driver of illness in neurodegenerative disorders, cancers and other conditions.
Yet the available methods for tracking mitochondrial motions have had significant limitations. Most mitochondrial tracking studies have been in neurons, where mitochondria tend to move along lengthy nerve fibers (axons), and the simple one-dimensional tracking techniques used in such studies aren't applicable to other cell types. The general particle tracking methods used to follow mitochondria in non-neuronal cells also have been less than ideal.
"Standard particle tracking methods tend to assume spherical particles, whereas mitochondria are more tubular; moreover a mitochondrion often splits into two or fuses with another, and general particle tracking methods don't account for that," Kandel said.
To devise a better method, Eckmann and his team started with an existing software application, ImageJ, published by the National Institutes of Health. They used it to turn raw videos of fluorescently-labeled mitochondria within whole skin cells into sharply resolved frames showing just the white mitochondria of one cell against a black background. They then applied a custom image-processing algorithm with object-recognition to the frames, in order to track individual objects as they moved slightly from frame to frame, and also to detect when they fused with or split off from other mitochondria.
The researchers initially employed the technique to look broadly at mitochondrial motions within skin cells. Prior studies in non-neuronal cells, using other methods, had suggested that mitochondria mostly move semi-randomly, with some showing brief spurts of directed motion presumably aimed at parts of the cell where their energy supply is needed. The Penn scientists found something different: a smoother distribution of mitochondrial motility on the whole-cell level, specifically what is known as a log normal distribution. "The cutoff between directed motions and more diffusive motions seems to be much less clear than had been thought," Kandel said.
Eckmann's laboratory has been investigating how disease or toxin-related disturbances to the structural elements of cells lead to disruptions of mitochondrial functions. He, Kandel and Chou therefore applied the new tracking method in further experiments along these lines.
In one experiment, they used a chemical to unravel railroad-like cellular structures called microtubules, along which mitochondria typically move—and found, expectedly, a marked decrease in net mitochondrial motility.
However, when they used a different compound to decompose actin microfilaments, which make up the basic "skeleton" structure of cells, they found that mitochondrial motility on average increased. "It's striking because it implies that actin microfilaments normally impede mitochondria," Kandel said. "It's definitely a point for further investigation."
Eckmann's laboratory is now following up with similar experiments tracking mitochondrial motility in other healthy and diseased cell types, including cancer cells. The new image-processing method is published online as a free scientific resource.