Epstein-Barr virus vaccine elicits potent neutralizing antibodies in animals
Researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and their collaborators have developed an experimental, nanoparticle-based vaccine against Epstein-Barr virus (EBV) that can induce potent neutralizing antibodies in vaccinated mice and nonhuman primates. Microscopic particles, known as nanoparticles, are being investigated as potential delivery vehicles for vaccines. The scientists' findings suggest that using a structure-based vaccine design and self-assembling nanoparticles to deliver a viral protein that prompts an immune response could be a promising approach for developing an EBV vaccine for humans.
First identified in 1964, EBV is one of the most common human viruses in the world, infecting nine out of 10 people at some point in their lives. Most people experience no illness or only mild symptoms. Most commonly spread through saliva, it is best known as the major cause of infectious mononucleosis, or mono. Worldwide, EBV is also associated with nearly 200,000 annual cases of cancer, including Burkitt and Hodgkin lymphoma, non-Hodgkin lymphoma, and stomach and nasopharyngeal cancers. Currently, there is no licensed vaccine to prevent EBV infection.
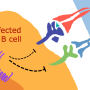
Most efforts to develop a preventive EBV vaccine have focused on glycoprotein 350, or gp350, a molecule on the surface of EBV that helps the virus attach to certain immune system cells called B cells. EBV gp350 is thought to be a key target for antibodies capable of preventing virus infection. Previously, scientists showed that vaccinating monkeys with gp350 protected the animals from developing lymphomas after exposure to a high dose of EBV. However, in the only large human clinical trial of an experimental EBV vaccine conducted to date, the EBV gp350 vaccine did not prevent EBV infection, but did reduce the rate of infectious mononucleosis by 78 percent.
To build on these findings, the researchers designed a nanoparticle-based vaccine that expressed the cell-binding portion of gp350. In their testing, the experimental vaccine induced potent neutralizing antibodies in both mice and nonhuman primates. Further, the investigational vaccine induced up to 100-fold higher levels of neutralizing antibodies in mice compared with previous vaccine designs by using structure-based design to precisely target the cell-binding site on gp350, the vulnerable part of the virus. The researchers believe the nanoparticle vaccine design could be used to create or redesign vaccines against other pathogens for which it has been difficult to induce effective immunity.
More information: M Kanekiyo et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor Binding Site. Cell DOI: 10.1016/j.cell.2015.07.043 (2015).