Prioritising the gene faults behind bowel cancer
As tumours develop, faults can pop up in what seems like a bewildering number of genes. And each time one cell becomes two, these mistakes can change and become more diverse.
But in some cases, the early events that lead to cancer can be readily identified. And for bowel cancer, scientific fingers have been pointing at one particular gene for almost 30 years.
Discovered by Cancer Research UK scientists back in the late 1980s, faulty copies of the adenomatous polyposis coli gene – shortened to APC – are fundamental, being found in around eight out of 10 cases of bowel cancer.
When faults in APC occur in the cells lining the bowel, they trigger a barrage of growth signals, causing the cells to multiply uncontrollably. This lays the foundations for bowel tumours, and over the years, research has cemented the importance of APC faults in the disease's development.
And we recently blogged about important US research showing that that bowel cancer cells remain reliant on APC faults to keep growing, even in the later stages of the disease.
But while we know that APC is important, what happens next? For some time researchers have debated the exact route through which faulty APC causes its striking effect. But this month, a new study from Professor Owen Sansom and his team at our Beatson Institute in Glasgow has lit up one route in particular.
And crucially, the team's findings refocus attention on a set of molecules that could one day be targeted with treatments.
Beta testing
The discovery centres on a molecule found in our cells called β-catenin, which can travel into a cell's nucleus and switch on particular genes that control how cells grow.
In normal bowel cells, one of APC's key jobs is to keep a cell's β-catenin levels in check by sending surplus amounts to the cell's internal waste disposal unit.
So if APC becomes damaged, β-catenin levels increase, exposing cells to relentless signals telling them to keep growing. Collectively, these signals are called the Wnt pathway, and they play an important role in how bowel cancer develops.
So the idea that APC faults cause bowel cancer by allowing β-catenin to escape being broken down, switching on Wnt signals that cause cells to multiply, is attractive in its (relative) simplicity.
But APC is a complex beast, and does much more inside a cell than just control β-catenin levels. It can also help modify a cell's internal skeleton, as well as the machinery that helps a cell divide – all of which could play a part in how faulty APC triggers cancer.
So in their latest study, our Glasgow team set about testing just how important APC's control over β-catenin and the Wnt pathway is in bowel cancer.
A cellular mop
The bowel is divided into two parts – the small bowel (small intestine) and the large bowel (colon and rectum). Previous research in mice has shown that switching APC off in bowel cells causes tumours to form in both the small and large bowel.
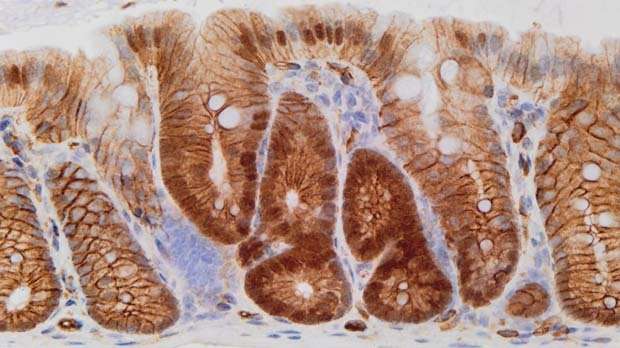
So to see if losing APC triggers bowel cancer through β-catenin, the team tested whether engineering cells to contain a faulty, active version of β-catenin would also cause tumours.
While these faults are much less common in human cancer than those in APC, they allow β-catenin to dodge APC's control and build up inside the cells where it enters the nucleus to switch on Wnt signals.
But only a few of the mice with the active version of β-catenin showed early signs of tumours, and it took much longer for them to form. And importantly, the growths only appeared in the small bowel, not the large bowel.
This was a surprising result, and told the team that there was something different about the large bowel tissue when β-catenin rather than APC was faulty, and that this was somehow protecting these cells from becoming cancerous.
A rummage through some previous scientific papers pointed the finger at a molecule called E-cadherin.
E-cadherin is a sticky protein, found on cells' surfaces, which helps hold neighbouring cells together. But research has suggested that it might also help mop up excess amounts of β-catenin inside cells – potentially neutralising the cancer-causing signals triggered by β-catenin entering the cell's nucleus.
The series of events
To test this theory, the team measured E-cadherin levels in the mice and, sure enough, they found they were much higher in the large bowel compared to the small bowel. And these increased levels of E-cadherin were indeed mopping up the excess β-catenin, preventing tumours from developing in the bowel.
Interestingly, early experiments from the team also suggest that there are crucial differences in E-cadherin and β-catenin in the human large bowel compared to the small bowel too.
Next, they engineered the mouse cells so that they produce slightly less E-cadherin than normal, to see how this might affect tumour formation. This produced another striking result.
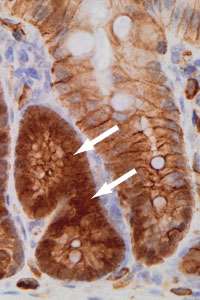
"When we reduced E-cadherin in the mice with faulty β-catenin, they quickly developed tumours in both the small and large bowel – just like when APC is faulty," explains David Huels, lead author on the study.
"With less E-cadherin to mop it up, the hyperactive β-catenin was free to enter the nucleus, switching on the growth signals that cause the tumours to grow."
And that's what you can see in the image on the right, with the brown colour showing β-catenin inside the cells. The darkest brown (marked with the arrows) is where faulty β-catenin is flooding the nuclei of these bowel cells, leading to the formation of tumour.
"This provides us with really good evidence that faulty APC is triggering bowel cancer through the Wnt pathway, and that other faults in the system can be mopped up – as with the faulty β-catenin," David explains.
"And crucially, this is the area that has attracted the most attention in terms of developing drugs that could switch these signals off again."
But the findings could also prove important for other cancers too.
The best route
Faults in β-catenin aren't that common in human bowel cancer – appearing in an estimated five in every 100 cases, compared to APC faults which occur in around 80 in 100 cases.
But these faults are much more common in liver cancer, with an estimated one in five cases carrying a fault in β-catenin similar to those our Glasgow team studied. And for a rare type of pancreatic cancer, called a solid pseudopapillary tumour, nearly all cases documented carry one of these faults.
So could changes to E-cadherin's mopping skills have a role to play in these tumours? David and the team think the answer might be yes.
When they looked at tissue samples from a small number of these tumours they found there were reduced levels of E-cadherin. "What this tells us is that the faulty β-catenin in these tissues has somehow outplayed the E-cadherin to form a tumour," says David.
"It's still early days, but all together our findings reinforce just how important the Wnt pathway is in cancer. Our next steps will be to see what combinations of these faults are required to kick start these tumours in people, and whether drugs targeting these signals can stop this."
By meticulously piecing together how these molecules interact, David and the rest of Professor Sansom's team are offering new insights into what fuels bowel cancer.
And with these priorities in order, the best route to beating bowel cancer is becoming clearer than ever before.
More information: "Comprehensive molecular characterization of human colon and rectal cancer." Nature 487, 330–337 (19 July 2012) DOI: 10.1038/nature11252
"Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration." Genes & Dev. 2004. 18: 1385-1390 DOI: 10.1101/gad.287404 Huels, D., et al. (2015).
"E-cadherin can limit the transforming properties of activating beta-catenin mutations." The EMBO Journal. DOI: 10.15252/embj.201591739