Scripps first in region to implant new defibrillator approved for use with MRI scans
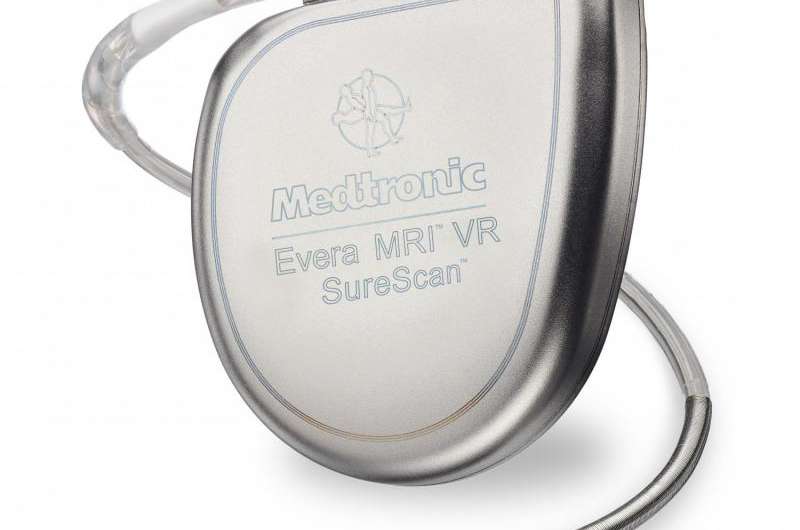
Scripps Health is the first health care provider in San Diego County to use the only implantable cardioverter defibrillator (ICD) device approved for use with magnetic resonance imaging (MRI) scans.
The milestone is an important one for patients who rely on ICDs to detect irregular heartbeats and deliver life-saving therapy to restore a normal rhythm. Until now, patients with earlier generations of ICDs have not been able to receive MRI scans or have been limited to obtaining an MRI as part of a research study.
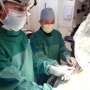
"There is no way to predict who will need an MRI," said Scripps Clinic cardiologist John Rogers, M.D., who implanted the new device in a patient Wednesday evening. "We are grateful to have this new technology that allows ICD patients to receive an MRI of any area of their body without limitation or concern about troublesome interactions."
An ICD is a small implantable heart device that is placed under the skin, typically just below the collarbone on the left side of the chest. For patients at risk for a life-threatening cardiac arrhythmia or sudden cardiac arrest, ICDs may be prescribed to continuously monitor heart rate and deliver an electrical signal to correct a life-threatening heart rate, if detected.
MRI is an imaging test used regularly for a wide range of diagnoses, including conditions such as stroke; cancer; Alzheimer's disease; and muscle, bone and back pain.
The Evera MRI SureScan ICD System from Medtronic was approved on Sept. 14 by the federal Food and Drug Administration to allow for MRI scans on any body part. The system includes design enhancements that allow it to safely undergo full-body MRIs, while maintaining the same longevity, proven shock reduction and physical size and shape of the previous versions of the device.
FDA approval was based on safety and efficacy data from the randomized, controlled Evera MRI Clinical Trial.
Dr. Rogers implanted the new device in Stacey Buchholtz, 49, of Carlsbad, who suffered a severe heart attack 14 months ago.
On Sept. 20, Buchholtz went to the emergency department at Scripps Memorial Hospital Encinitas after experiencing chest pain and a rapid heartbeat. She was transferred to Scripps Green Hospital in La Jolla where she underwent a series of tests and heart monitoring by Dr. Rogers.
After monitors recorded an episode of ventricular tachycardia, a potentially life-threatening fast heart rhythm, Dr. Rogers prescribed the Evera MRI ICD.
"Given my medical history, there is a good chance I will need to undergo scans in the future," Buchholtz said. "It's great that the Evera MRI ICD allows me to have access to the best MRI scans available."
Ranked No. 1 for heart care in San Diego County and No. 19 nationally by U.S. News and World Report, Scripps is also the largest provider of heart medical services in the region, caring for more than 76,000 cardiovascular patients every year. Its newly opened Prebys Cardiovascular Institute brings together leading researchers, physicians, staff and technologies in the most advanced center dedicated to heart care on the West Coast.