Rescuing intestinal stem cells from attack in type 1 diabetes
Up to 80 percent of people with long-standing type 1 diabetes develop gastrointestinal symptoms—abdominal pain, bloating, nausea, vomiting, diarrhea, constipation and fecal incontinence—that severely diminish quality of life. Research at Boston Children's Hospital now reveals the cause of this complication, known as diabetic enteropathy, and a possible prevention and treatment strategy.
The study, led by Paolo Fiorina, MD, PhD, demonstrates how diabetes can destroy the stem cells that maintain the intestinal lining—through excess production of a hormone called insulin-like growth factor binding protein 3 (IGFBP3). In animal models, a protein that "soaks up" this hormone was able to restore normal intestinal stem cell function. The findings appear in the October 1 issue of Cell Stem Cell, accompanied by an editorial commentary.
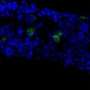
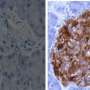
Fiorina and colleagues began by studying 60 patients with long-standing type 1 diabetes. Through proteomics analysis of the patients' blood, they discovered strikingly elevated levels of IGFBP3—almost five times that in 20 healthy controls. Levels of the hormone corresponded with GI symptoms and colonic stem cell abnormalities, and rose dramatically in correspondence with blood sugar levels.
"Everyone has IGFBP3, but in people with hyperglycemia, the liver makes more of it," explains Fiorina, a physician and scientist in the Division of Nephrology at Boston Children's. "We think that the liver senses the high blood glucose level and makes more of this hormone try to reduce glucose absorption in the intestine."
IGFBP3—dubbed "enterostaminine" by the investigators—may be necessary to health, as it checks excess intestinal stem-cell proliferation, says Fiorina. Unfortunately, in type 1 diabetes, this protective mechanism is on overdrive.
A recombinant drug?
Turning to a mouse model of diabetes, Fiorina and colleagues showed that IGFBP3 binds to a receptor in the intestines called TMEM219 and disrupts intestinal stem cell function. When they cloned a portion of the TMEM219 protein and gave it to the mice, it bound up the circulating IGFBP3, causing levels to drop and healthy stem cell function to resume. In diabetes patients who had kidney-pancreas transplantation, which restores normal blood glucose, IGFBP3 levels and stem cell function normalized and GI symptoms diminished.
Fiorina and colleagues are now doing further tests on the TMEM219-derived drug to see if it could prevent or treat diabetic enteropathy. They are also testing ways to stop liver cells from producing so much enterostaminine to begin with.
Scientifically, the findings represent one of the first known instances of a hormone controlling stem cell production. They suggest that stem cells' regenerative qualities can be exploited without having to actually manipulate and deliver cells or figure out how to prevent foreign cells from being destroyed by the immune system.
"If you have hormones like enterostaminine that actually affect the stem cell niche, you can bypass cell therapy and allow the niche to do its job in a better way," says Fiorina. "This approach could reshape the way we imagine cell therapy. I think that many other hormones with similar functions will be discovered."
Fiorina is also exploring new treatments for diabetes itself. Under an agreement with Fate Therapeutics, his lab will investigate the ability of both genetically engineered and pharmacologically modulated blood stem cells to curb the autoimmune activity that destroys pancreatic beta cells. Thus far, their mouse data indicate that the genetically engineered cells home to the pancreas, reduce abnormal immune-cell activity and reverse hyperglycemia (see abstract 26-OR in this PDF) and that cells modulated with drugs have the same immune-regulating properties as the genetically engineered cells.